How could you prepare the following ketones by reaction of an acid chloride with a lithium diorganocopper
Question:
How could you prepare the following ketones by reaction of an acid chloride with a lithium diorganocopper reagent?
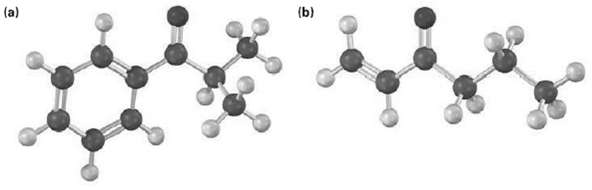
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Two combinations of acid chloride ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could you prepare the following compounds with benzene as one of the starting materials? (a) (b)
-
How could you prepare the following amides using an acid chloride and an amine or ammonia? (a) CH3CH2CONHCH3 (b) N, N-Diethylbenzamide (c) Propanamide
-
a. Starting with isopropylacetylene, how could you prepare the following alcohols? 1. 2-methyl-2-pentanol 2. 4-methyl-2-pentanol b. In each case a second alcohol would also be obtained. What alcohol...
-
[10 marks] Click the link below to see the code for a program. Your task is as in assignment 3: re-write the code of this program so that, to a user of the program, it works as before, but in your...
-
Even with the popularity of online job-search sites, traditional job-search techniques are still important. What are some traditional sources for finding jobs?
-
What is a hazard quotient, and how is it used in risk assessment?
-
How is the financial plan related to the other parts of a firms overall strategic plan? AppendixLO1
-
A contract calls for annual payments of $1,200. Find the present value of the contract, assuming that (1) The number of payments is 7 and the current interest rate is 6 percent: (2) The number of...
-
Question 1) You invest $100,000 today in an online savings account for 5 years. If the interest rate is 4% p.a., calculate the future value under each of the following scenarios: a) Interest rates...
-
1. After reviewing her personal automobile policy, Bronwyn realized that she had $75,000 of single-limit, Part A coverage; $15,000 of Part B coverage; $75,000 of Part C coverage; and "full" Part D...
-
Write the mechanism of the reaction just shown between 3, 4, 5-trimethoxybenzoyl chloride and morpholine to form trimetozine. Use curved arrows to show the electron flow in each step.
-
Write the mechanism of the reaction between p-hydroxyaniline and acetic anhydride to prepare acetaminophen.
-
Tri-State Megabucks Lottery advertises a $10 million grand prize. The winner receives $500,000 today and 19 annual payments of $500,000. A lump sum option of $5 million payable immediately is also...
-
Popcorn company is expected to pay $1 dividend per share at the end of this year, $1.50 dividend per share at the end of year 2, $2 dividend per share at the end of year 3, and $2.50 dividend per...
-
Increased spending for COVID economic relief is an important issue for many struggling in the current economy. A specific policy to combat this issue is put forward and it is found that 78% of...
-
James worked a total of 186 hours for the month of June 2020. His rate per hour is working hours of the 450 per hour. Overtime premium is 30%. The company is 8 hours a day. The company's regular...
-
Question Researchers collected a simple random sample of 36 children who had been identified as gifted in a large city. The following histograms show the distributions of the IQ scores of mothers and...
-
Shown below is activity for one of the products of Denver Office Equipment: January 1 balance, 700 units @ $55 per unit $38,500 Purchases: January 10: 700 units @ $60 per unit January 20: 1,100 units...
-
identify sources and uses of funds to assist in the creation of a statement of cash flows.
-
A sample statistic will not change from sample to sample. Determine whether the statement is true or false. If it is false, rewrite it as a true statement.
-
According to MO theory, which molecule or ion has the highest bond order? Highest bond energy? Shortest bond length? 2- 02, 0, 0
-
Indicate whether the following peaks in the mass spectrum of 1-heptanol are odd-electron or even-electron ions. (a) m/z = 83 (b) m/z = 70 (c) m/z = 56 (d) m/z = 41
-
Calculate the energy in kJ mol-1 of the light described in Problem 12.1(b) Blue light with = 4800 A
-
The mass spectrum of methyl isobutyl ether does not show a peak due to inductive cleavage, in contrast to the mass spectrum of di-sec-butyl ether (Eq. 12.31). Use what you know about carbocation...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


