How could you use 1H NMR to distinguish between the following pairs ofisomers? (a) CH3CH=CHCH2CH3 and CH2
Question:
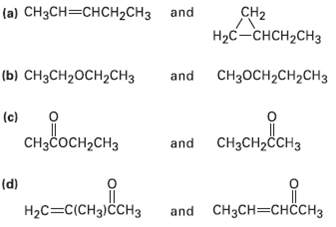
How could you use 1H NMR to distinguish between the following pairs ofisomers?
Transcribed Image Text:
(a) CH3CH=CHCH2CH3 and CH2 Hас— снсH2сHз (ы) CнзсH20CH2CHз and CH3OCH2CH2CH3 (c) CHзCосн,сHз and CH3CH2ČCH3 (d) Hас—CICHз)СсHз and CH3CH=CHÖCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 47% (17 reviews)
First check each isomer for structural differences that are obviously recognizable in the H NMR spec...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could you use 1H NMR spectroscopy to distinguish among the following esters? 018 18 - CH3 OH
-
How would you use 1 H NMR spectroscopy to distinguish between the following compounds? (a) (b) (c) (d) (e) (f) . . . CI
-
How could you use IR spectroscopy to distinguish between the following pairs of isomers? a. b. c. (CH3CH2)3N and (CH3CH2CH2)2NH 0 CH.CCH,CH, and CH CHCH-CH, CH3 CHCHO and CH CHOCH
-
1. Give 3 examples of well-defined sets. 2. Name two subsets of the set of whole numbers using both the listing method and the rule method. 3. Let B [1, 3, 5, 7, 9}. List all the possible subsets of...
-
Canon has manufactured high quality camera since it was founded in 1933. SLR-cameras (i.e., not point and shoot cameras) are purchased in two parts: the body and the lenses. Photographers who want a...
-
A proton is fired from far away toward the nucleus of an iron atom. Iron is element number 26, and the diameter of the nucleus is 9.0 fm. What initial speed does the proton need to just reach the...
-
List guidelines for effective trainers. AppendixLO1
-
Provide a short explanation of the difference between each of the following sets of terms: a. Bound control versus unbound control b. Design mode versus run mode c. Symbol versus symbol on a forms...
-
For the just completed year, Hanna Company had net income of $144,000. Balances in the companys current asset and current liability accounts at the beginning and end of the year were as follows:...
-
Suggest a reasonable explanation for each of the following observations: (a) The second-order rate constant k for saponification of ethyl trifluoroacetate is over 1 million times greater than that...
-
The acid-catalyzed dehydration of 1-methylcyclohexanol yields a mixture of two alkenes. How could you use 1H NMR to help you decide which waswhich? CH CH - 0* CH2
-
Propose structures for compounds with the following formulas that show only one peak in their 1H NMR spectra: (a) C5H12 (b) C5H10 (c) C4H8O2
-
In order to write the null and alternative hypotheses for a single-sample t test, the researcher needs to know whether the test has one or two ____.
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
Distinguish between reliability and validity?
-
Repeat the previous problem, but close the positions on September 20. Use the spreadsheet to find the profits for the possible stock prices on September 20. Generate a graph and use it to identify...
-
Silver nitrate solutions are often used to plate silver onto other metals. What is the maximum amount of silver (in grams) that can be plated out of 4.8 L of an AgNO 3 solution containing 3.4% Ag by...
-
Aluminum trichloride (AICI3) dissolves in ether with the evolution of a large amount of heat. (In fact, this reaction can become rather violent if it gets too warm.) Show the structure of the...
-
Show how you would accomplish the following transformations. Some of these examples require more than one step. (a) 2-methylpropene 2, 2-dimethyloxirane (b) 1-phenylethanol 2-phenyloxirane (c)...
-
The 2001 Nobel Prize in Chemistry was awarded to three organic chemists who have developed methods for catalytic asymmetric syntheses. An asymmetric (or enantioselective) synthesis is one that...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...

Study smarter with the SolutionInn App


