How could you use Diels?Alder reactions to prepare the following products? Show the starting diene and dienophile
Question:
How could you use Diels?Alder reactions to prepare the following products? Show the starting diene and dienophile in each case.
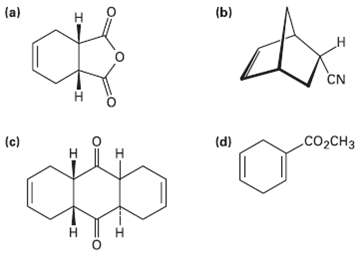
Transcribed Image Text:
(a) (b) н CN сооCнз (c) (d) н H. н т
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
First find the cyclohexene ring formed by the DielsAlder ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could you use 1H NMR spectroscopy to distinguish among the following esters? 018 18 - CH3 OH
-
How could you use expectancy theory to increase your own motivation level?
-
How could you use standard process costing to obtain information that helps improve the efficiency of the process?
-
An examiners close inspection of the annual financial statements and the accounting records revealed that Mawani Inc. may have violated some accounting principles. The examiner questioned the...
-
Discuss how well you think Starbuck's tuition reimbursement program meets the criteria for selecting employee benefits (organizational objectives, employees' expectations and values, and benefits...
-
What is the common name for NIST SP 800-12? What is the documents purpose? What resources does it provide?
-
Discuss the merits of the different types of planning.
-
Kaitlin Carlton, a CPA sole practitioner, prepares tax returns each year for approximately 100 clients. Items 1 through 8 each represent an independent factual situation in which Kaitlin has prepared...
-
a. Explain how managers incentive to smooth income is consistent with the bonus plan hypothesis. b. Explain whether or not smooth income can be viewed as a forecast of future sustainable income. c....
-
Based in Vancouver, BC, Glide Ltd. is a manufacturer of snowboards for the Canadian market. It has two models: the "FreeRide" and the "FreeStyle". Glide is attempting to determine how many units...
-
1, 3-Pentadiene is much more reactive in Diels?Alder reactions than 2, 4-pentadienal. Why might this be? H 1,3-Pentadiene 2,4-Pentadienal
-
Aldrin, a chlorinated insecticide now banned for use in the United States, can be made by Diels?Alder reaction of hexachloro-1, 3-cyclopentadiene with norbornadiene. What is the structure of aldrin?...
-
Open the Translator Solution (Translator Solution.sln) file contained in the VB2015\Chap07\Translator Solution-Sub folder. The application should use three independent Sub procedures to translate the...
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
Business Analytics Application: Run a stepwise discriminant analysis using the twelve perception variables from the customer survey database. Prepare a brief report on your findings.
-
A bar of length = 1 has one fixed and one free end and stiffness function c(x) = 1 - x. Find the displacement when subjected to a unit force. Pay careful attention to the boundary condition at the...
-
A solution contains a mixture of substance A and substance B, both of which are volatile. The mole fraction of substance A is 0.35. At 32 C the vapor pressure of pure A is 87 mmHg, and the vapor...
-
Propose a mechanism for the acid-catalyzed reaction of benzaldehyde with methanol to give benzaldehyde dimethyl acetal.
-
Propose a mechanism for the acid-catalyzed hydrolysis of cyclohexanone dimethyl acetal.
-
Why were no products from McLafferty rearrangement observed in the spectrum of butan-2-one (Figure 18-3)? In Figure 18.3 100 43 80 0 60 CH-C CH2CH 40 20 57 0 10 20 30 40 50 60 70 80 90 100 110 120...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


