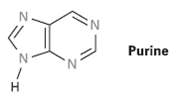
How many electrons does each of the four nitrogen atoms in purine contribute to the aromatic ?
Question:
How many electrons does each of the four nitrogen atoms in purine contribute to the aromatic ? system?
Transcribed Image Text:
N. Purine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (20 reviews)
H H N H Purine Purine is a tenxelectron aromatic ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How many electrons does each of the following elements have in its outermost electron shell? (a) Magnesium (b) Molybdenum (c) Selenium
-
How many valence electrons does each of the following atoms have? (a) Na (b) Cl (c) Si (d) B (e) Ne (f) N
-
How many electrons does a Ba atom have to lose to have a complete octet in its valence shell?
-
Consider the following velocity distribution curves A and B. a. If the plots represent the velocity distribution of 1.0 L of He(g) at STP versus 1.0 L of Cl2(g) at STP, which plot corresponds to each...
-
Suppose you work in the HR department of a company that wants to hire production workers as independent contractors. What advice would you give management about this idea?
-
A building owner is evaluating the following alternatives for leasing space in an office building for the next five years: Net lease with steps. Rent will be $15 per square foot the first year and...
-
Choose a popular, independent, local restaurant. Write a two-page description of that property that you feel defines its trade dress. AppendixLO1
-
Sage Learning Centers was established on July 20, 2016, to provide educational services. The services provided during the remainder of the month are as follows: July 21. Issued Invoice No. 1 to J....
-
Depreciation Method Straight-line Declining-balance Salvage Useful Life Cost Value in Years $ 96,000 $ 6,000 5 110,000 10,000 4 Bus Acquired 1 1/1/15 2 1/1/15 The company depreciate the first bus at...
-
PROFESSIONAL SUMMARY: 7+ years of experience in developing and implementing Web applications using .NET and Microsoft Technologies including C#, Net Core, .Net Framework, WCF, XML, Microservices and...
-
Azulene, a beautiful blue hydrocarbon, is an isomer of naphthalene. Is azulene aromatic? Draw a second resonance form of azulene in addition to thatShown. Azulene
-
Give IUPAC names for the following substances (red = O, blue =N): (a) (b)
-
What are the purposes and the key components of an RIA?
-
What are major initiatives would you expect to see in a strategic plan focusing on a public health organization?
-
The purchase of \(\$ 500\) of supplies on account will: a. Increase both assets and stockholders' equity by \(\$ 500\) b. Increase assets and decrease liabilities by \(\$ 500\) c. Increase assets and...
-
Venus Company owned a service truck that was purchased at the beginning of 2011 for \(\$ 20,000\). It had an estimated life of three years and an estimated salvage value of \(\$ 2,000\). Venus uses...
-
You are observing the sales department staff using exponential smoothing to fore- cast monthly sales. Their forecast for January's sales was 12,000 units. January's actual sales figure became...
-
Use the ID3 algorithm to build the full decision tree for the data set given in Section 10.9.2. 10.9.2 Example We will start with the training data given below: Film Country of origin Big star Genre...
-
understand the advantages of using replacement cost (where that is possible), carry out the procedures for using replacement cost and show how its use leads to a more useful calculation of the...
-
In Exercises 1-2, rewrite each verbal statement as an equation. Then decide whether the statement is true or false. Justify your answer. 1. The logarithm of the difference of two numbers is equal to...
-
Consider the reaction of A to form B: A reaction mixture at 298 K initially contains [A] = 0.50 M. What is the concentration of B when the reaction reaches equilibrium? 2 A(g) = B(g) Kc = 1.8 x 105...
-
Raffinose is a trisaccharide (C18H32O16) isolated from cottonseed meal. Raffinose does not reduce Tollens reagent, and it does not mutarotate. Complete hydrolysis of raffinose gives D-glucose,...
-
Cellulose is converted to cellulose acetate by treatment with acetic anhydride and pyridine. Cellulose acetate is soluble in common organic solvents, and it is easily dissolved and spun into fibers....
-
Cytosine, uracil, and guanine have tautomeric forms with aromatic hydroxyl groups. Draw these tautomeric forms.
-
The payroll register of Ruggerio Co. indicates $13,800 of social security withheld and $3,450 of Medicare tax withheld on total salaries of $230,000 for the period. Federal withholding for the period...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...

Study smarter with the SolutionInn App


