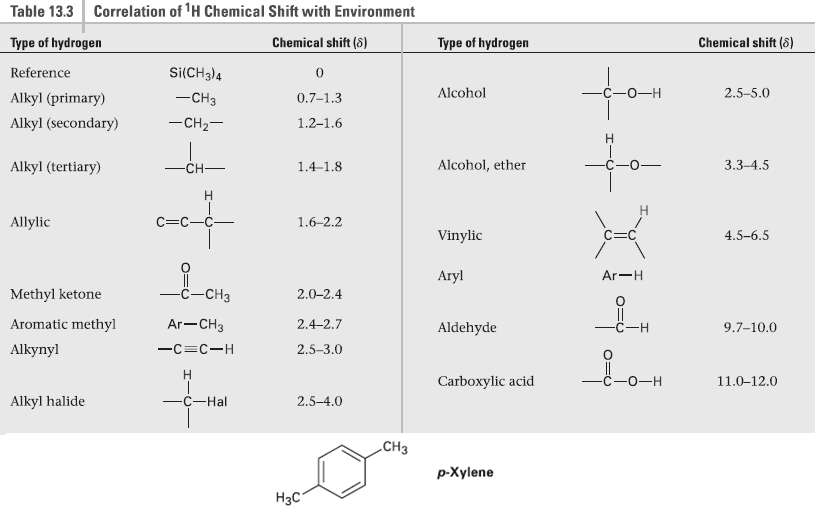
How many peaks would you expect in the 1H NMR spectrum of 1, 4-dimethyl-benzene para-xy1ene or p-xylene)?
Question:
How many peaks would you expect in the 1H NMR spectrum of 1, 4-dimethyl-benzene para-xy1ene or p-xylene)? What ratio of peak areas would you expect on integration of the spectrum? Refer to Table 13.3 for approximate chemical shifts, and sketch what the spectrum would look like. (Remember from Section 2.4 that aromatic rings have two resonanceforms.)
Transcribed Image Text:
Correlation of 'H Chemical Shift with Environment Table 13.3 Type of hydrogen Chemical shift (8) Chemical shift (8) Type of hydrogen Si(CH3)4 Reference —С -о—н Alcohol 2.5-5.0 -CH3 Alkyl (primary) 0.7-1,3 -CH2- Alkyl (secondary) 1.2-1.6 for H. 3.3-4.5 -CH- Alcohol, ether Alkyl (tertiary) 1.4-1.8 C=C-C- Allylic 1.6-2.2 Vinylic 4.5-6.5 Aryl Ar-H -c-CH3 Methyl ketone -CHз 2.0-2.4 Ar-CH3 Aromatic methyl 2.4-2.7 9.7-10.0 Aldehyde C-H 2.5-3.0 Alkynyl -C=C-H С -о—н Carboxylic acid 11.0-12.0 -C-Hal Alkyl halide 2.5-4.0 СНз p-Xylene Нас
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (23 reviews)
HC CH3 pXylene There are two absorptions in the H ...View the full answer

Answered By

ARCHANA BISHT
I had Completed my M.Sc. from Banaras Hindu University. After that I cleared CSIR NET exam two times and GATE (MA) exam. I started online tutoring on various websites and I had an experience of 4 years. I also worked in an academy for 1 years where I help the students in the preparation of CSIR NET and GATE.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How many signals would you expect in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) (d) (e) (f) CI Br -
-
How many peaks would you expect to see on the strip chart after amino acid analysis of bradykinin? Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg Bradykinin
-
The 1H NMR spectrum of 1-chloropentane is shown at 60 MHz (spectrum H) and 500 MHz (spectrum I), Explain the differences in appearance of the two spectra, and assign the signals to specific hydrogens...
-
The profit before tax as reflected in the draft statement of comprehensive income of Sword Limited for the financial years ended 31 December 2020 and 31 December 2021 respectively was as follows:...
-
How do you think the equilibrium in question 15 will change if cross-price elasticity's of demand increase? How would you alter the equation to show such an increase? Can you compute the new...
-
What are the strength and direction of the electric field at the position indicated by the dot in FIGURE EX23.2? Specify the direction as an angle above or below horizontal. (+ 3.0 nC 5.0 cm |10 cm +...
-
Answer the following questions using the data from the Excel file Toys Unlimited.xlsx from Problem 16.49. a. Forecast Toys Unlimited sales for each quarter next year using a multiple regression with...
-
When Teris outside basis in the TMF Partnership is $80,000, the partnership distributes to her $30,000 cash, an account receivable (fair market value of $60,000, inside basis to the partnership of...
-
The Spartan Corporation has engaged in several transactions listed below: 1. Sold food and beverages for cash 2 Purchased investments 3. Exchanged its common stock for long-term bonds 4. Sold common...
-
Gale Company has the following inventory and purchases during the fiscal year ended December 31, 2020. Gale Company employs a perpetual inventory system. Required 1. Calculate the dollar value of...
-
Identify the different kinds of non-equivalent protons in the following molecule, and tell where you would expect each toabsorb. CH-CH C
-
Predict the splitting patterns you would expect for each proton in the followingmolecules: (b) CH2H2Br (a) CHBr2CH3 (c) CICH,CH2CH2CI (e) CHCH-CHCH (f) (d) CHCH2CH CH ondeo CH
-
Consider the circuit in Figure 10.40(a). The transistor and circuit parameters are the same as given in Problem 10.85 except for the width-to-length ratios of the transistors. Determine the \(W / L\)...
-
1. Define a person-centered model of care in LTC facilities. 2. Describe two leadership behaviors and two leadership qualities most conducive to moving long-term care organizations toward more...
-
question 5 all parts 8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin...
-
3- Define and describe, in detail, the various communication styles as they relate to negotiation and conflict resolution. Compare the advantages and disadvantages of the styles. Provide a detailed...
-
SJ Corp ahs the following data for 2020: RM, beginning of 5,000; Purchases of raw materials is 50,000; return of defective raw materials to suppliers of 4,000; return of direct materials from the...
-
A company is issuing $340,000 worth of 4-year bonds on October 8, 2023, bearing an interest rate of 2%, payable annually. Assume that the current market rate of interest is 3%. a) Will the bonds be...
-
Understand the key principles of sampling in business research.
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
An aqueous solution containing 17.5 g of an unknown molecular (nonelectrolyte) compound in 100.0 g of water has a freezing point of -1.8 C. Calculate the molar mass of the unknown compound.
-
Convert the following infrared wavelengths to (a) 6.24 (m, typical for an aromatic (b) 3.38 (m, typical for a saturated bond (c) 5.58 (m typical for a ketone carbonyl (d) 5.75 (m typical for an ester...
-
All of the following compounds absorb infrared radiation between 1600 and In each case, 1. Show which bonds absorb in this region. 2. Predict the approximate absorption frequencies. 3. Predict which...
-
Describe the characteristic infrared absorption frequencies that would allow you to distinguish between the following pairs of compounds. (a) 2,3-dimethylbut-2-ene and 2,3-dimethylbut-1-ene (b)...
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


