How would you use the reaction of an amide with LiAlH4 as the key step is going
Question:
How would you use the reaction of an amide with LiAlH4 as the key step is going from bromo cyclohexane to (N, N-dimethylaminomethyl) cyclohexane? Write all the steps in the reactionsequence.
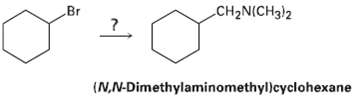
Transcribed Image Text:
Br CH2N(CH3)2 (N,N-Dimethylaminomethyl)cyclohexane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
Br Mg ether CHNCH32 1 LIAIH4 2 HO MgBr OC 1 CO ether 2 HO NCH32 CH32NH NaOH OC SOCI O...View the full answer

Answered By

MICHAEL KICHE
I was employed studypool for the first time in tutoring. I did well since most of my students and clients got the necessary information and knowledge requested for. I always submitted the answers in time and followed the correct formatting in answering eg MLA or APA format,
Again I worked with the writers bay where I did writing and got many clients whom we worked with so closely. They enjoyed every single service I delivered to them. My answers are always correct.
4.70+
13+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you use the net marketing contribution for a company like Clorox to forecast net profit and earnings per share?
-
How would you use the graphic on this chapter's first page and to assess Apple's 2010 marketing ROS (27.5%) and marketing ROI (323%)?
-
How would you use the control variate approach to improve the estimate of the delta of an American option when the binomial tree approach is used?
-
(a) Employing the intercept technique, determine the average grain size for the steel specimen whose microstructure is shown in Figure (a); use at least seven straight-line segments. (b) Estimate the...
-
What is a customized résumé, and why should you have one?
-
Suppose the market for household drinking water in San Francisco is modeled as follows: S = MSC = 10 + 0.2Q D = MSB = 40 0.4Q, where Q is millions of gallons per day and MSC and MSB are in cents per...
-
STOCK SPLIT After a 5-for-l stock split, Strasburg Company paid a dividend of $0 75 per new share, which represents a 9% increase over last years pre-split dividend. What was last years dividend per...
-
Grand Champion, Inc., purchased Americas Sweethearts Corporation on January 1, Year 1. At the time, Americas Sweethearts had $750,000 of identifiable assets and $525,000 of liabilities. Grand...
-
the record book or computer program where accounting data is first entered
-
Yoko Iwabuchi is the controller of Kondo, Inc., an electronic controls company located in Osaka. She recently attended a seminar on activity-based costing (ABC) in Tokyo. Kondos traditional cost...
-
How would you convert N-ethylbenzamide to each of the following products? (a) Benzoic acid (b) Benzyl alcohol (c) C 6 H 5 CH 2 NHCH 2 CH 3
-
Write the mechanism of the reaction shown in figure between coenzyme A and acetyl adenylate to give acetylCoA. NH2 CH "3 HSCH-CH2NHCCH2CH2NHCCHCCH,0P - - Phosphopantetheine 2-O3PO Adenosine...
-
Why is lead found in all deposits of uranium ore?
-
REQUIRED: Cost of production report under the following assumptions: Lost units - normal, discovered at the beginning Lost units - normal, discovered at the end Lost units - abnormal, discovered when...
-
ABC, Inc., manufactures only two products: Gadget A and Gadget B. The firm uses a single, plant wide overhead rate based on direct-labor hours. Production and product-costing data are as follows:...
-
.Jean Saburit has gone over the financial statements for Saburit Parts, Inc. The income statement has been prepared on an absorption costing basis and Saburit would like to have the statement revised...
-
When a constant force is applied to an object, the acceleration of the object varies inversely with its mass. When a certain constant force acts upon an object with mass 2 kg, the acceleration of the...
-
Use the following for all 3 circuits. V1 = 9.0 V, V = 12.0 V R = 2.0 ohms, R = 4.0 ohms, R3 = 6.0 ohms, R4 = 8.0 ohms C1 = 3.0 C = 3.0 (a) Find I in circuit A (b) Find I1 in circuit B R w R3 V R R4...
-
Utilize the product cost percentage and contribution margin methods of menu pricing.
-
Economic feasibility is an important guideline in designing cost accounting systems. Do you agree? Explain.
-
Draw an MO energy diagram and predict the bond order of Li 2 + and Li 2 . Do you expect these molecules to exist in the gas phase?
-
The ==C-H stretching absorption of 2-methyl- I-pentene is observed at 3090 cm-1. If the hydrogen were replaced by deuterium, at what wavenumber would the ==C-D stretching absorption be observed?...
-
Which of the following vibrations should be infrared-active and which should be infrared inactive (or nearly so)? (a) (b) (c) (CH, ),C-=0 C-O stretch C C stretch (CHC CI C-Cl stretch
-
What magnetic field at the proton would be required to cause "spin flipping" in an NMR experiment in which the frequency imposed on the sample is 900 MHz?
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


