Identify the asymmetric carbon(s) (if any) in each of the following molecules. ,
Question:
Identify the asymmetric carbon(s) (if any) in each of the following molecules.
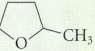
Transcribed Image Text:
СН,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
The asymmet...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify the primary, secondary, and tertiary carbon atoms and the hydrogen atoms in each of the following molecules: (a) Ethane; (b) Pentane; (c) 2-methylbutane; (d)...
-
Identify all of the asymmetric carbon atoms in each of the following structures. (a) (b) (c) , , H C CH CH2OH NH2 CH3
-
Indicate the asymmetric carbon atoms in the following compounds: (a) (b) CH3 CH3 CH2-CH-CH-C NH2 NH2 Br Br
-
50 Kg of ammonium sulphate (NH4)2SO4 and 30 Kg of urea CO(NH2)2 fertilizers were applied in two equal sizes of plots A and B to enrich their nitrogen content. Show by working which plot was more...
-
What are the principal factors that can be varied in setting credit policy?
-
Cite and discuss the attributes of a successful sales manager. Which one do you consider to be the most important? Why?
-
5. Pop uses the equity method of accounting for its investment in Son. REQuIRED 1. Prepare a schedule showing Pops income from Son for the years 2016, 2017, and 2018. 2. Compute Pops net income for...
-
A Kubota tractor acquired on January 9 at a cost of $75,000 has an estimated useful life of 20 years. Assuming that it will have no residual value, determine the depreciation for each of the first...
-
6 points Question 14 On June 1, Ava, Inc. borrowed $500,000 cash from Broadway Bank under a six month noninterest-bearing note, with a 6% dincount rate. Interest was payable at maturity a. (2 pts) i...
-
Jill Beaver was an employee at RGIS Inventory Specialists. She became ill while on vacation with acute sinusitis, bronchitis, and an ear infection. She was prescribed an antibiotic by a physician,...
-
Draw the structures of all compounds with the formula C6H12C12 that can exist as meso compounds. Indicate how many meso compounds are possible for each structure.
-
(a) Draw sawhorse projections of ephedrine (Problem 6.32) about the C1-C2 bond for all three staggered and all three eclipsed conformations. (b) Examine each conformation for chirality. How do the...
-
What are the differences among an examination, a review, and agreed- upon procedures?
-
Hardwick Corporation manufactures fine furniture for residential and industrial use. The demand for the company's products has increased tremendously in the past three years. As a result, the company...
-
Problem 3: Use the product rule to find the following derivatives. Leave your answer in the form f'(x)g(x)+ f (x) g' (r). That is, do not simplify. (a) s(t)=t3 cos (t) (b) F(y): = (12-1) (v + 5 y)...
-
Do an internet search of two or three organizations in your field of study (Human Resources). Review the organization or business and its hiring practices using some of the questions from the...
-
4. A process was set to meet the design specifications of USL = 26 and LSL = 18. The standard deviation of the process was found to be 1.2. The process mean was set to 22.5. a) Calculate the process...
-
Evaluate the broad environment, e.g., political, social, legal, in which the industry of OCSIP is located. How does this affect the industry?
-
When constructing an amortization schedule, how is the periodic payment amount calculated?
-
Consider the sections of two circuits illustrated above. Select True or False for all statements.After connecting a and b to a battery, the voltage across R1 always equals the voltage across R2.Rcd...
-
Calculate the standard enthalpy difference between the cis and trans isomers of 2-butene. Specify which stereoisomer is more stable. The heats of formation are, for the cis isomer, 27.40 kJ mol 1 ,...
-
Give the structure of: (a) (E)-4-allyl-1,5-octadiene (b) (2E,7Z)-5-[(E)-1-propenyl]-2,7-nonadiene Be sure to read Study Guide Link 4.2 if you have difficulty with this problem.
-
What is the configuration (E or Z) of the following alkene? (The numbers and letters are for reference in the solution.) H 2 HC C=C 02 CHCHCBrCH3 61 62 CHCHCH3 T CH3 6,6-dibromo-3-isobutyl-2-heptene
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


