Identify the indicated hydrogens in the following molecules as pro-R or pro-S: (c) (a) (b)
Question:
Identify the indicated hydrogen’s in the following molecules as pro-R or pro-S:
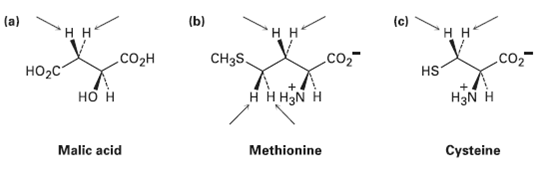
Transcribed Image Text:
(c) (a) (b) нн нн нн CH3S, .co2- Соон Но-с HS нннаN н H3N H но н Cysteine Malic acid Methionine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
a proS HOC proR COH HO ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify the indicated protons in the following molecules as unrelated, homotopic, enantiotopic, ordiastereotopic: (a) (b) Cysteine
-
Identify the carbon atoms in the following molecules as primary, secondary, tertiary, orquaternary: H H CH CH CHCH2CHCH2CH Gs (c) (a) (b) CH2H2CH CHCCH2CCH CH
-
Label carbon in the following molecules as primary, secondary, tertiary, or quaternary.
-
On January 1, 2018, Rastall Co. signed a long-term finance lease for an office building. The terms of the lease required Rastall to pay $30,000 annually, beginning December 31, 2018, and continuing...
-
Most often we say that force causes acceleration. But when Evan took a ride in a rocket sled at Bonneville Salt Flats, blood was forced to the back of his brain, nearly blacking him out. Discuss and...
-
A sealed box contains a monatomic ideal gas. The number of gas atoms per unit volume is 5.00 10 20 atoms/cm 3 , and the average translational kinetic energy of each atom is 1.80 10 -23 J. (a) What...
-
Review one of the sample RFPs available from www.techsoup.org/toolkits/rfp or another RFP for an IT project. Write a paper summarizing the purpose of the RFP and how well you think it describes the...
-
Dell Inc. and Hewlett-Packard Company (HP) compete with each other in the personal computer market. Dell's primary strategy is to assemble computers to customer orders, rather than for inventory....
-
Harry Warrington is the CEO of Hellshire distribution company and is confused regarding the treatment of Research and Development expenditures. Define both terms and explain to him the difference in...
-
Tire pressure monitoring systems (TPMS) warn the driver when the tire pressure of the vehicle is 26% below the target pressure. Suppose the target tire pressure of a certain car is 28 psi (pounds per...
-
Answer Problem 9.61 for the epoxidation of trans-4-octene. CH3CH2CH2CH=CHCH2CH2CH3 CH3CH2CH2CH-CHCH2CH2CH3 RCO3H 4-Octene 4,5-tane
-
Identify the indicated faces in the following molecules as Re orSi:
-
The lowest frequency in the audible range is 20 Hz. (a) What are the lengths of (a) the shortest open-open tube and (b) the shortest open-closed tube needed to produce this frequency?
-
Companies that invest heavily in eco-friendly initiatives, such as transitioning to renewable energy sources or implementing carbon offset programs, may initially face increased operational costs....
-
Answer each question individually please. 14-13 What are the advantages and drawbacks of universities using social media to communicate with various stakeholdersstudents, potential students, alumni,...
-
act as a consultant hired by the operations director of the Barry Computer Company provide a financial analysis and comparison to the industry. You will conduct a financial ratio analysis to gain a...
-
Building a sense of community is not just a moral thing to do, but also a pragmatic one. In today's competitive and ever-evolving business environment, the organizations that can attract the most...
-
Watch https://youtu.be/U3MtvvNjUR4 What do you think of Dr. Saint's ideas about barriers to change? What do you think about social learning? Could this tool be used to make real change? How can the...
-
What role do committees play in the club management structure? LO.1
-
Sandcastles, Inc.s management has recently been looking at a proposal to purchase a new brick molding machine. With the new machine, the company would not have to buy bricks. The estimated useful...
-
Find the mass of barium metal (in grams) that must react with O 2 to produce enough barium oxide to prepare 1.0 L of a 0.10 M solution of OH .
-
Give the principal organic product(s) expected when 2- methylthiophene or other compound indicated reacts with each of the following reagents. (a) HNO3 (b) Dilute aqueous NaOH (c) Product of part (a)...
-
Give the principal organic product(s) expected when 2- methylpyridine or other compound indicated reacts with each of the following reagents. (a) Diliute aqueous NaOH (b) HNO3, H2SO4, heat; then -OH...
-
Rank the following compounds in order of increasing reactivity toward nitration with HNO3 and explain your choices: thiophene, benzene, 3-methylthiophene, and Z-methvlfuran.
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


