Identify the most acidic hydrogen in each of these compounds: a) HOCCH,CH,SOH 0 CO H 0
Question:
Identify the most acidic hydrogen in each of these compounds:
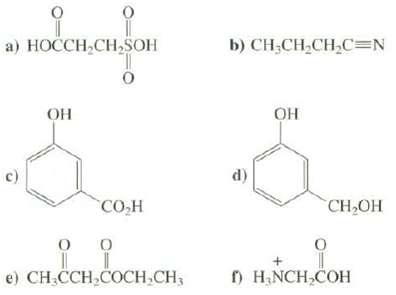
Transcribed Image Text:
요 a) HOCCH,CH,SOH ОН 0 CO H 0 I e) CH₂CCH₂COCH₂CH3 0 b) CH-CH,CH,C=N d) ОН 0 CH₂OH f) H₂NCH₂COH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a A sulfonic acid is a stronger acid than a carboxylic acid HOCCHCHSO TH b The circled hydrogen is ...View the full answer

Answered By

Payal Mittal
I specialize in finance and accounts.You can ask any question related to til undergradution.Organizational behaviour and HRM are my favourites for you can always relate to them and is an art with practical knowledge base.
4.90+
226+ Reviews
778+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which is the most acidic hydrogen in each of these compounds? (a) H 2 NCH 2 CH 2 OH (b) CH 3 CH 2 OH (c) CH 3 SH
-
Identify the most acidic proton in each of the following compounds: Xo Xx
-
Identify the most acidic proton in each of the following compounds and explain your choice: (a) (b) 0-H F3C 0-H CI. CI
-
What responsibilities can a Crew Boss delegate to a subordinate supervisor? (Select all that apply) Re-supplying crew and equipment Communicating crew wake up time for next operational period...
-
Apollo Can Ltd manufactures recyclable soft drink cans. A unit of production is a box of 12 cans. The following standards have been set by the production engineering staff and the factory accountant:...
-
NGW, a consumer gas provider, estimates a rather cold winter. As a result it decides to enter into a futures contract on the NYMEX for natural gas on November 2, 2016. The trading unit is 10,000...
-
P 5-5 Upstream inventory sale, 100 percent owned On February 20, 2012, Angel AG acquired all common stock of Mark AG. The book value of Mark AGs net assets was equal to fair value at the acquisition...
-
Distinction between relevance and cost behavior Stanley Company makes and sells a single product. Stanley incurred the following costs in its most recent fiscal year. Stanley could purchase the...
-
What is the difference in tax paid on a product worth $19,562 in Ontario vs Alberta. (HST in Ontario is 13% and GST in Alberta is 5%) Question 3 options: 1430 1615 1495 1565
-
Design a database for a world-wide package delivery company (e.g., DHL or FedEX). The database must be able to keep track of customers (who ship items) and customers (who receive items): some...
-
Complete these equilibrium reaction sin the most reasonable manner possible using the curved arrow convention to show the movement of electrons in the reactions, Predict whether the reactants or the...
-
Show the products of these acid-base reactions and predict whether the equilibria favor the reactants or the products: a) CHCCHCCHCH + OCHCH b) CHCHNO + CHO: CH3 (c) CH3COCH, + CHCH- 10 1:Z: LL CH,...
-
Iva Holz is a licensed incorporated CPA. During the first month of operations of her business, the following events and transactions occurred: May 1 Invested \(\$ 42,000\) cash in exchange for common...
-
Woodland Wearables produces two models of smartwatches, the Basic and the Flash. The watches have the following characteristics:Basic Flash Selling price per watch$ is 270$ 460 Variable cost per...
-
Based on the information provided and recognizing the value of coordinating across its portfolio of businesses, how should LendingTree manage these newer businesses? * as more integrated units * as...
-
Trust Fund Worksheet Background An inter vivos trust was created by Isaac Posney. Isaac owned a large department store in Juggins, Utah. Adjacent to the store, Isaac also owned a tract of land that...
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Gracia Enterprises operates across five industries. Task 1 : After reviewing the information provided, determine which of the five operating segments are reportable based on the revenue test, asset...
-
Explain the managerial functions required in energy in the Management of energy.
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
Prove the identity. sin( x) = sin x
-
Compound A, MW = 86, shows an IR absorption at 1730 cm1 and a very simple 1H NMR spectrum with peaks at 9.7 (1 H, singlet) and 1.2 (9 H, singlet). Propose a structure for A.
-
Compound B is isomeric with A (Problem 19.63) and shows an IR peak at 1715 cm1. The 1H NMR spectrum of B has peaks at 2.4 (1 H, septet, J = 7 Hz), 2.1 (3 H, singlet), and 1.2 (6 H, doublet, J =...
-
The 1 H NMR spectrum shown is that of a compound with formula C 9 H 10 O. How many double bonds and/or rings does this compound contain? If the unknown has an IR absorption at 1690 cm ?1 , what is a...
-
Prepare journal entries to record the following events: Jul. 1 Klemens Company accepted a 5%, 3-month, $8,000 note dated July 1 from Mox Company for the balance due on Mox's account. Jul. 31 Klemens...
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...

Study smarter with the SolutionInn App


