In light of the fact that tertiary alkyl halides undergo spontaneous dissociation to yield a carbocation plus
Question:
In light of the fact that tertiary alkyl halides undergo spontaneous dissociation to yield a carbocation plus halide ion, propose a mechanism for the followingreaction:
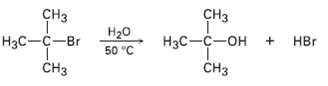
Transcribed Image Text:
CHз сHз Нзс—с—он + НBr CHз Нзс —с—Br Нао 50 "C CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
CH3 HCCBr CH...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In view of the fact that hot air rises, why does it generally become cooler as you climb a mountain? (Note that air has low thermal conductivity.)
-
In view of the fact that hot air rises, why does it generally become cooler as you climb a mountain? (Note that air has low thermal conductivity.)
-
In Example 26.2, Janets handling of the fact that her data showed 26 volunteers in the control group when there should have been only 25.
-
The balance sheet data for Alans Lightworks, Corp., at August 31, 2012, and September 30, 2012, follow: Requirement 1. The following are three independent assumptions about the business during...
-
Sketch graphs for the relationships described in each of the following problems and select one or more of the families of functions discussed in Section 2.4.4 to represent it. 1. The relationship...
-
Chicken eggs possess a hard, porous shell of calcite mineral. Cylindrical pores of 10 micron (µm) diameter running through the 0.5 mm thickness of the shell permit the exchange of gases to the...
-
Identify the major characteristics of a corporation. LO5
-
Bella Floral Designs is a wholesale shop that sells flowers, plants, and plant supplies. The transactions shown below took place during January. DATE TRANSACTIONS Jan. 3 Sold a floral arrangement to...
-
Using the appropriate interest table, compute the present values of the following periodic amounts due at the end of the designated periods. $ 50,570 receivable at the end of each period for 9...
-
35. Consider the argument: Premise 1: p v-q Premise 2: - q Conclusion: P Which truth values of p and g is the argument, written as a conditional, true? i. pis false; q is true il. pis true; q is...
-
Tertiary alkyl halides, R 3 CX, undergo spontaneous dissociation to yield a carbocation, R 3 C + , plus halide ion. Which do you think reacts faster, (CH 3 ) 3 CBr or H 2 C = CHC (CH 3 ) 2 Br?...
-
Carboxylic acids (RCO 2 H; p K a 5) are approximately 10 11 times more acidic than alcohols (ROH; pK a 16). In other words, a carboxylate ion (RCO 2 ) is more stable than an alkoxide ion (RO )....
-
Explain the impact of inflation on rates of return.
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
Downward communication is normally initiated by employees who are lower in the organizations hierarchy. A. True B. False
-
You work as an operations consultant for a textile company. Your client has a well-established distribution system in the US market. The company has hundreds of stores and four distribution centers....
-
Draw the Lewis structure for urea, H 2 NCONH 2 , one of the compounds responsible for the smell of urine. (The central carbon atom is bonded to both nitrogen atoms and to the oxygen atom.) Does urea...
-
Which of the following pairs of compounds could be separated by recrystallization or distillation? (a) meso-tartaric acid and (±)-tartaric acid (HOOC-CHOH-CHOH-COOH) (b) (c) (d) CH,CH, O CH,CH...
-
To show that (R)-2-butyl (R, R)-tartrate and (S)-2-butyl (R,R)-tartrate are not enantiomers, draw and name the mirror images of these compounds.
-
The following four structures are naturally occurring optically active compounds. Star the asymmetric carbon atoms in these structures. CHO H CH, COOH OH OH H,N H serine erythrose menthol camphor
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


