Ketones react with alcohols to yield products called acetals. Why does the allcis isomer of 4-tert-butyl-l, 3-cyclohexanediol
Question:
Ketones react with alcohols to yield products called acetals. Why does the allcis isomer of 4-tert-butyl-l, 3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal while other stercoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal. Use molecular models for help.
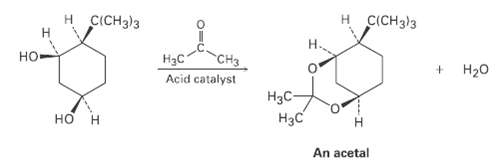
Transcribed Image Text:
H CICH3)3 н CICH)3 но H3C CH3 Acid catalyst H20 H3C- Нас но An acetal
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Draw the four possible isomers of 4tertbutylcyclohexane13diol Make models of these isomers als...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw the more stable chair conformation for each of the following compounds: CI Cl Cl
-
Draw the more stable chair conformation for each of the following compounds: (a) -d-Galactopyranose (b) -d-Glucopyranose (c) -d-Glucopyranose
-
Draw the more stable chair conformation of -daltropyranose and label all substituents as axial or equatorial.
-
Suppose a state was trying to decide whether to fund primary and secondary education with a property tax or an income tax. What implications might this choice have for land use in the state?
-
Three years ago you purchased a Heinz corporate bond that pays 3.125 percent annual interest. The face value of the bond is $1,000. What is the total dollar amount of interest that you received from...
-
Apple Inc. (AAPL) designs, manufactures, and markets personal computers and related software. Apple also manufactures and distributes music players (iPod), mobile phones (iPhone), and smartwatches...
-
Refer to Exercise E-1 and for each of the March transactions identify the journal in which it would be recorded. Assume the company uses a sales journal, purchases journal, cash receipts journal,...
-
Of the ineffective resolution examples, which was the worst, in your opinion? Why?
-
! Required information Excel Analytics 12-1 (Static) Quality Cost Report (LO12-2] (The following information applies to the questions displayed below.) Harvey Company designs and produces surgical...
-
You decided to run an experiment - improve current CTA on the in-app pricing page (1 experimental variation and one control group). Each month the pricing page is seen by 16,000 users. 800 of those...
-
Amantadine is an antiviral agent that is active against influenza A infection and against some strains of H5N1 avian flu. Draw a three-dimensional representation of amantadine showing the chair...
-
Alcohols undergo an oxidation reaction to yield carbonyl compounds on treatment with CrO 3 . For example, 2-tert-butylcyclohexanol gives 2-tert-butylcyclo- hexanone. If axial ?OH groups are generally...
-
The Robinson-Patman Act was enacted by Congress to prevent price discrimination among competitors. What three defenses for price cuts are available to the seller under the Act? LO2
-
You have two dashboards in the same workspace named Production and Manufacturing. Your company's Power BI administrator creates the following two dashboard data classifications: Medium Impact (MEDI)...
-
Question 2: Red Rocks Corporation was organized on September 1. Red Rocks encountered the following events during the first month of operations. a. Received $65,000 cash from the investors who...
-
he previous three weeks of data is below for the sales of sheds at SHEDS INC. Calculate the forecast for the next perioud (week 4) using a two period weighted moving average using weights of 3 and 2....
-
/3 3) ST tan(x) - In(cosx) dx What is the value of u? us dulcis) What is the corresponding value of du? du= 1-5mx dx cosx You must show all of your work in the space below to earn full credit. 9/3 So...
-
Please use the file which provides the data to answer the problems 1-3. Problem 1) The time Students entered the classroom of OM 390, Introductory Operations Management, was recorded by the professor...
-
What are the advantages and disadvantages of conducting surveys online?
-
Why is it necessary to study the diffusion of molecules in biological systems?
-
For each of the following substances describe the importance of dispersion (London) forces, dipoledipole interactions, and hydrogen bonding: (a) HCl; (b) Br 2 ; (c) ICl; (d) HF; (e) CH 4 .
-
Azulene has an appreciable dipole moment. Write resonance structures for azulene that explain this dipole moment and that help explain its aromaticity.
-
(a) The iSH group is sometimes called the mercapto group. 6-Mercaptopurine is used in the treatment of acute leukemia. Write its structure. (b) Allopurinol, a compound used to treat gout, is...
-
Explain how 13C NMR spectroscopy could be used to distinguish the ortho-, meta-, and para-dibromobenzene isomers one from another.
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
Which of the following statement regarding a post-closing trial balance is not true
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions

Study smarter with the SolutionInn App


