Lactate, a product of glucose catabolism in oxygen-starved muscles, can be converted into pyruvate by oxidation. What
Question:
Lactate, a product of glucose catabolism in oxygen-starved muscles, can be converted into pyruvate by oxidation. What coenzyme do you think is needed? Write the equation in the normal biochemical format using a curvedarrow.
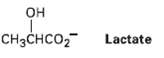
Transcribed Image Text:
OH Lactate CHзснCO2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
OH CH3CHCO Lactate NAD NADHH ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What market research techniques do you think is most effective and least effective? Are there conditions under which some market research techniques work better than others? If so, what are those...
-
What type of business strategy do you think is most compatible with a high-performance work system? Why?
-
What social problem do you think is the worlds biggest? Wars? Global warming? Resource imbalances? How could you start to solve a big social problem through marketing?
-
5.In a nut shell, when you look back over time, the power of image has always been there. Even in the prehistoric era, they used imagery to communicate, and what's even more incredible is that we are...
-
Note the enterprise and equity valuations for Life Technology in the Excel spreadsheet model entitled Target Valuation Model on the companion website accompanying this book. View this as the base...
-
What are some of the best practices in establishing a payroll system?
-
For indicator categories that are not significant, collapse the categories with the reference category. (How are you handling the category with the 0.083 p-value?) Rerun the logistic regression with...
-
A saturated-vapor mixture of maleic anhydride and benzoic acid containing 10 mol% acid is a by-product of the manufacture of phthalic anhydride. This mixture is distilled continuously at 13.3 kPa to...
-
Adams, Peters, and Blake share profits and losses for their APB Partnership in a ratio of 2:3:5. When they decide to liquidate, the balance sheet is as follows: Assets Cash Adams, Loan Other Assets $...
-
The three-element springs and dashpot model shown is subject to a creep experiment. Show how the length (or strain) increases with time. At time t the stress is removed. Show how the sample recovers....
-
Why arent the glycolysis and gluconeogenesis pathways the exact reverse of one another?
-
How many moles of acetyl CoA are produced by catabolism of the following substances? (a) 1.0 mol glucose (b) 1.0 mol palmitic acid (c) 1.0 mol maltose
-
Compute the probability of 10 successes in a random sample of size n = 16 obtained from a population of size N = 70 that contains 24 successes.
-
Gulf Shore Lawn and Garden Maintenance provides two general outdoor services: lawn maintenance and garden maintenance. The company charges customers $18.0 per hour for each type of service, but lawn...
-
Two level sections of an east highway (G=0) are to be connected. Currently, the two sections of highway are seperated by a 4000-ft (horizontal distance), 2% grade. The westernmost section of highway...
-
A solution contains 2 x 10-3 moles Ca2+/L and 3 x 10-4 moles Mg2+/L. Given the formation constants for CaEDTA2- and MgEDTA2- of 1010.6 and 108.7, respecively, calculate: 1) Concentration of MgEDTA2-...
-
The direct material (DM) price variance is $2,650 favorable and the DM usage variance is $3,000 unfavorable. The budgeted amount of DM for each unit of product is 2 lbs. to be purchased at the...
-
On January 1, 2023, AMI Corporation purchased the non-cash net assets of Oriole Ltd. for $8,399,900. Following is the statement of financial position of Oriole Ltd. from the company's year- end the...
-
Describe three essential elements in the decision-making process.
-
Make an argument that Williams had a right to delay the closing until after August 1.
-
One of the purposes of the kidneys is to transfer useful chemicals from the urine to the blood, and toxins from the blood to the urine. In the transport of glucose from the urine to the blood, the...
-
Acid-catalyzed dehydration of 2,2-dimethyl-1-hexanol gave a number of isomeric alkenes including 2-methyl-2-heptene as shown in the following formula. (a) Write a stepwise mechanism for the formation...
-
Compound A (C4H10) gives two different monochlorides on photochemical chlorination. Treatment of either of these monochlorides with potassium tert-butoxide in dimethyl sulfoxide gives the same alkene...
-
Compound A (C6H14) gives three different monochlorides on photochemical chlorination. One of these monochlorides is inert to E2 elimination. The other two monochlorides yield the same alkene B...
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


