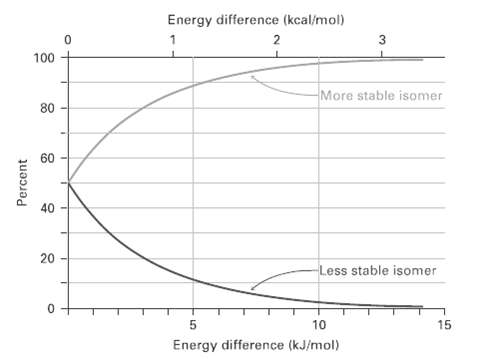
Look at Figure, and estimate the percentages of axial and equatorial conformers present at equilibrium inbromo-cyclohexane. Energy
Question:
Look at Figure, and estimate the percentages of axial and equatorial conformers present at equilibrium inbromo-cyclohexane.
Transcribed Image Text:
Energy difference (kcal/mol) 2 100 More stable isomer 80 60 20 Less stable isomer 10 15 Energy difference (kJ/mol) Percent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Table shows that an axial bromine causes 2 x 10 kJmol of steric strain ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Look again at textbook Figure 2.2. Trace it onto a piece of scrap paper. Now draw two demand curves on your figure. Draw one that is horizontal at P = $32/MWH and one that is vertical at quantity =...
-
Look at Figure in Chapter 2, which tracks median P/E ratios from 1963 to 2003. Explain why P/E ratios were low in the 1970s and high in the 1960s and 1990s.
-
Look at figure 3.1 on page 60. Precisely which of the six Is were used in case file 3? Which ones were used in case file 1? Were any used in case file 2? INOCULATION One goal of these procedures is...
-
Determine whether the following code fragment takes linear time, quadratic time, or cubic time (as a function of \(n\) ). for (int i = 0; i < n; i++) for (int j = 0; j < n; j++) j) C[i][j] 1.0; if (i...
-
Mark Crane purchased a $1,000 corporate bond five years ago for $1,060. The bond pays 4.5 percent annual interest. Five years later, he sold the bond for $950. Calculate the total return for Mr....
-
Beginning inventory, purchases, and sales for Item 88-HX are as follows: Assuming a perpetual inventory system and using the last-in, first-out (LIFO) method, determine (a) The cost of goods sold on...
-
When special journals are used, where are cash payments by check recorded? AppendixLO1
-
The adjusted trial balance of Business Reduction Systems at March 31, 2016, follows: Requirements 1. Journalize the required closing entries at March 31, 2016. 2. Set up T-accounts for Income...
-
Pat started a new business on September 1, 2022. He is self-employed. He drove 500 business miles from September 1 December 31, 2022. He qualifies to use the standard mileage rate. What amount will...
-
A chemical process product produces two main products (Delta and Echo) and one by-product (Golf) from a joint product (Beta). None of the products require processing after the split-off point....
-
Why do you suppose an axial cyano (CN) substituent causes practically no 1, 3-diaxial steric strain (0.4 kJ/mol)? Use molecular models to help with your answer.
-
Draw the most stable chair conformation of the following molecules, and estimate the amount of strain in each: (a) trans-1-Chloro-3-methylcyclohexane (b) cis-1-Ethyl-2-rnethylcyclohexane (c)...
-
Distinguish between liquid and illiquid assets, and list some assets that are liquid and some that are illiquid.
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
The determination of standards for direct material, direct labor, variable manufacturing overhead and fixed manufacturing overhead. LO.1
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
A vapor volume of 1.17 L forms when a sample of liquid acetonitrile, CH 3 CN, absorbs 1.00 kJ of heat at its normal boiling point (81.6 C and 1 atm). What is vap H in kilojoules per mole of CH 3 CN?
-
The following reaction does not produce the product shown. (a) Predict the major product from the conditions shown above, and write a detailed mechanism for its formation. (b) What reaction...
-
Predict the products from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) Hl (excess) Hi H,so,,H20 O MeONa O MeOH, cat. H2SO (1) EtSNa (2) H2o HCl (1 equiv.) MeONa (1)EtON...
-
Provide the reagents necessary to accomplish the following syntheses. (a) (b) (c) (d) MeO MeO SEt SEt
-
C: The sor at the poopecin 0ieund to twe oxind places)
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...
-
Carla Vista Cart Inc. has the following information for 2026 : The rate of return on assets Carla Vista Cart Inc. is 81.06%17.27%30.58%14.00%

Study smarter with the SolutionInn App


