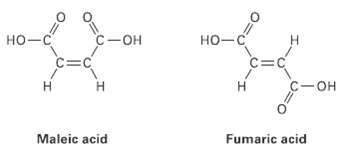
Maleic acid has a dipole moment, but the closely related fumaric acid, a substance involved in the
Question:
Maleic acid has a dipole moment, but the closely related fumaric acid, a substance involved in the citric acid cycle by which food molecules are metabolized, does not.Explain
Transcribed Image Text:
с — он но-с C-OH но-с C=C c=C С —он Maleic acid Fumaric acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (18 reviews)
In maleic acid the individual dipole moments ad...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A water molecule has a dipole moment of 6.3 x 10 30 C m. A sample contains 1021 molecules of water. Their dipole moments are all oriented in the direction of an electric field of 2.5 x 10 N/C....
-
The PF3 molecule has a dipole moment of 1.03 D, but BF3 has a dipole moment of zero. How can you explain the difference?
-
The ammonia molecule (NH3) has a dipole moment of 5.0 X 10-30 C m. Ammonia molecules in the gas phase are placed in a uniform electric field E with magnitude 1.6 X 106 N/c. (a) What is the change in...
-
A bank reconciliation takes time and must balance. An employee was struggling in balancing the bank reconciliation. Her supervisor told her to plug (make an unsupported entry for) the difference,...
-
Elaborate rules exist that require employers to prepay various types of Federal taxes. Summarize the major issues that an employer must resolve if it is to comply with the requirements?
-
Why are consumers so willing to rent from Redbox? How was Redbox able to overcome some of its earliest challenges? What are some recommendations for ways that Redbox can maintain its high market...
-
Using the PewSocialMedia dataset, address this question: does being married/ partnered have an effect on how upset you are over things that happen in your life? Run a t-test using the Index of...
-
Keystone Manufacturing Company started operations on January 1, 2019. During 2019, the company engaged in the following transactions: 1. Issued common stock for $40,000. 2. Paid $10,000 cash to...
-
Question 8 (of 8) > value: 5.00 points On April 1, 2010, Stone's Backhoe Co. purchases a trencher for $250,000. The machine is expected to last five years and have a salvage value of $25,000. Compute...
-
In a DS/BPSK system, the feedback shift register used to generate the PN sequence has length m = 19. The system is required to have an average probability of symbol error due to externally generated...
-
Assign formal charger to the atoms in each of the followingmolecules: ( NNEN: CH (c) HCN3DN-DN: (a) H3C-N-O: CH
-
Rank the following substances in order of increasing acidity: C CH3CH3 CHCCH2CCH Acetic acid (pKa = 4.76) Phenol Acetone (pKa = 19.3) 2,4-Pentanedione (pKa = 9) (pKa = 9.9) %3D %3D
-
Why is fair trade an ethical issue? What are the competitive advantages of fair trade? What are the potential problems with fair trade?
-
Select a qualitative research design to establish whether the strike by the federal workers in Canada may have been caused by a leadership
-
There is a single loop circuit with three resistors as follows. Take R-D 2, R= D/2 2 and R= D/3 2 and connect to 12 V battery. a. What can you say about the connection of R., R2 and Rs, Explain the...
-
1:This week we learned about Team Communication and Difficult conversation. Reflect on your own experience of a difficult conversation that you had to handle. Explain what happened and how you...
-
What is the value chain of Doordash? Name their primary and support activities.
-
Blue Water Clinic is a mobile field medical facility that has been offering Covid-19 vaccination services to rural communities in southwest Ontario under the supervision of medical authorities. With...
-
Which paragraph is most appropriate? a. Findings from internal audit reports and files play an important role. It is insulting to produce a report for management that only has supporting...
-
Write a paper about the Working relationship in the organization- collaboration within and outside the organization
-
a. Show that b. Use the result of part (a) to show that for a stable system at equilibrium ( V /T) S and ( V /T) P must have opposite signs. c. Two separate measurements are to be performed on a gas...
-
Draw all of the stereo isomers of 1, 2-dimethylcyclopropane. Explain which rotate plane-polarized light.
-
Explain whether these compounds rotate plane-polarized light: H a) HC-CCH, HC Br c) Br J. s CI b) Cl CI d) HC -CH3
-
Draw Fischer projections for these compounds? CHOH a) H-C-CI CH3 COH b) HC-OH A CH3 c) H CHCH CH CH3
-
Comparing the actual and planned cost of a consulting engagement completed by an engineering firm such as Allied Engineering.
-
What is the NPV of a project that costs $34,000 today and is expected to generate annual cash inflows of $11,000 for the next 7 years, followed by a final inflow of $14,000 in year 8. Cost of capital...
-
help!!! Use the above information to calculate ending inventory using FIFO for a company that uses a perpetua/inventory system

Study smarter with the SolutionInn App


