Method of continuous variation. Make a graph of absorbance versus mole fraction of thiocyanate from the data
Question:
Method of continuous variation. Make a graph of absorbance versus mole fraction of thiocyanate from the data in the table.
.png)
(a) What is the stoichiometry of the predominant Fe(SCN)n3-n species?
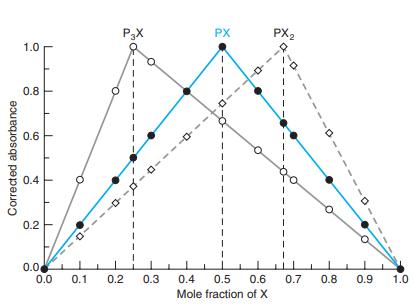
(b) Why is the peak not as sharp as those in Figure 18-8?
(c) Why does one solution contain 10.0 mM acid and the other 15.0 mM acid?
Figure 18-8
Transcribed Image Text:
mL Fe3 solution 30.00 27.00 24.00 21.00 18.00 15.00 12.00 9.00 6.00 3.00 mL SCN solution 3.00 6.00 9.00 12.00 15.00 18.00 21.00 24.00 27.00 30.00 Absorbance at 455 nm 0.001 0.122 0.226 0.293 0.331 0.346 0.327 0.286 0.214 0.109 0.002 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
a Maximum absorbance occurs at XSCN 0500 stoichiometry 1 1 ...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Simulating a Job's plot. Consider the reaction , for which K [AB 2 ]/[A][B ]2 . Suppose that the following mixtures of A and B at a fixed total concentration of 104M are prepared: (a) Prepare a...
-
Gold nanoparticles (Figure 16-29) can be titrated with the oxidizing agent TCNQ in the presence of excess of Br to oxidize Au(0) to AuBr in deaerated toluene. Gold atoms in the interior of the...
-
The variation of refractive index, n, with wavelength for fused silica is given by where is expressed in μm. (a) Make a graph of n versus λ with points at the following...
-
Suppose a town concludes that it costs on average $30.00 per household to manage the disposal of the waste generated by households each year. It is debating two strategies for funding this cost: (1)...
-
Jill Epp, a friend of yours, overheard a discussion at work about changes her employer wants to make in accounting for uncollectible accounts. Jill knows little about accounting, and she asks you to...
-
Assume that a governmental entity provides other postemployment benefits (OPEB) to its retirees. The entity commissions an actuarial valuation of the OPEB plan and contributes to a trust in...
-
12-5. Hertz cre su ventaja diferencial en los puntos de __________ en su auditora de contacto con el consumidor.
-
Valuing a right Knight inventory Systems, Inc., has announced a rights offer. The company has announced that it will take four rights to buy a new share in the offering at a subscription price of...
-
Come-Clean Corporation produces a variety of cleaning compounds and solutions for both industrial and household use. While most of its products are processed independently, a few are related, such as...
-
At a point upstream of the throat of a converging-diverging nozzle, the properties are V1 = 200 m/s, T1 = 300 K, and p1 = 125 kPa. If the exit flow is supersonic, compute, from isentropic theory, (a)...
-
Now we use Solver to find K for the previous problem. The only absorbing species at 332 nm is the complex, so, from Beer's law, [complex] = A/ (because pathlength = 1.000 cm). I 2 is either free or...
-
The indicator xylenol orange (Table 11-3) forms a complex with Zr(IV) in HCl solution. Prepare a Job plot from the data in the table and suggest the stoichiometry of the complex (xylenol orange) x Zr...
-
Each of the curves shown in Exercises 1 through 4 is the graph of one of the six functions listed here. In each case, match the given curve to the proper function. fi(x) = 2e-2x 2 f3(x) 1 - ex In x...
-
Name and define the more common constraints in any given project.
-
Graph the function f(x)=-x+4x-20 State where f(x) is increasing and decreasing. State any absolute extrema (if they exist). Determine the Domain and Range.
-
A residential wiring circuit is shown in the figure. In thismodel, the resistor R 3 is used to model a 250 V appliance(such as an electric range), and the resistors R 1 and R 2 are used to model 125...
-
1. The speed limit on some interstate highways is roughly 100 km/h. (a) What is this in meters per second? (b) How many miles per hour is this? 2. A car is traveling at a speed of 33 m/s. (a) What is...
-
Questions 33 and 34 are based on the following information: Bilog Company's budgeted fixed overhead costs are P50,000 and mthe variable factory overhead rate is P4 per direct labor hour. The standard...
-
What document establishes the start of construction time?
-
A Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an E2 process. In studying the stereochemistry of this elimination, it was found that...
-
Calculate the concentration of H 2 Y 2- at the equivalence point in Exercise 11-C. Exercise 11-C Calculate pCu 2+ (to the 0.01 decimal place) at each of the following points in the titration of 50.0...
-
Suppose that 0.010 0 M Mn 2+ is titrated with 0.005 00 M EDTA at pH 7.00. (a) What is the concentration of free Mn 2+ at the equivalence point? (b) What is the quotient [H 3 Y - ]/[H 2 Y 2- ] in the...
-
Suppose that 0.010 0 M Mn 2+ is titrated with 0.005 00 M EDTA at pH 7.00. (a) What is the concentration of free Mn 2+ at the equivalence point? (b) What is the quotient [H 3 Y - ]/[H 2 Y 2- ] in the...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...

Study smarter with the SolutionInn App


