The indicator xylenol orange (Table 11-3) forms a complex with Zr(IV) in HCl solution. Prepare a Job
Question:
The indicator xylenol orange (Table 11-3) forms a complex with Zr(IV) in HCl solution. Prepare a Job plot from the data in the table and suggest the stoichiometry of the complex (xylenol orange)xZrz.
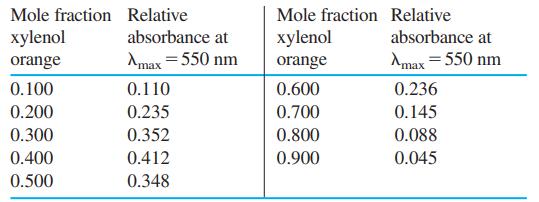
Table 11-3
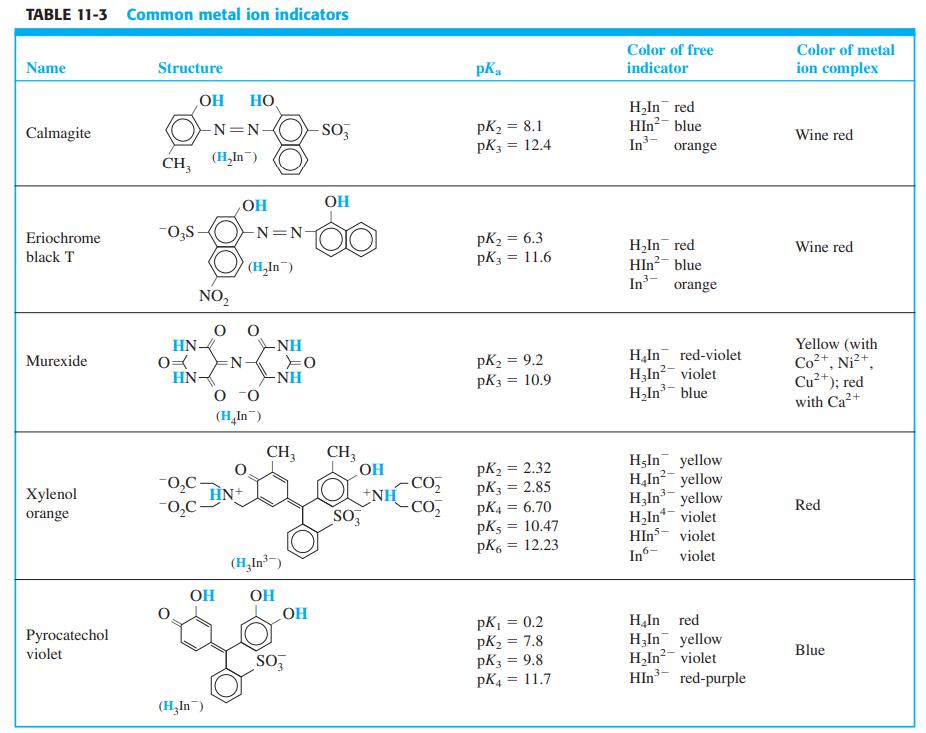
Transcribed Image Text:
Mole fraction Relative Mole fraction Relative xylenol absorbance at xylenol absorbance at orange Amax = 550 nm orange Amax = 550 nm 0.100 0.110 0.600 0.236 0.200 0.235 0.700 0.145 0.300 0.352 0.800 0.088 0.400 0.412 0.900 0.045 0.500 0.348
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)
The Job plot peak is at a xylenol orange mole fraction of 040 ...View the full answer

Answered By

Muhammad adeel
I am a professional Process/Mechanical engineer having a vast 7 years experience in process industry as well as in academic studies as a instructor. Also equipped with Nebosh IGC and lead auditor (certified).
Having worked at top notch engineering firms, i possess abilities such as designing process equipment, maintaining data sheets, working on projects, technical biddings, designing PFD and PID's etc.
Having worked as an instructor in different engineering institutes and have been involved in different engineering resrearch projects such as refinery equipment designing, thermodynamics, fluid dynamics, chemistry, rotary equipment etc
I can assure a good job within your budget and time deadline
4.90+
52+ Reviews
60+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The metal ion indicator xylenol orange (Table 11-3) is yellow at pH 6 ( max = 439 nm). The spectral changes that occur as VO 2+ is added to the indicator at pH 6 are shown here. The mole ratio VO 2+...
-
Iron (III) forms a complex with thiocyanate ion that has the formula Fe(SCN)2+. The complex has an absorption maximum at 580 nm. A specimen of well water was assayed according to the scheme below....
-
In the Kjeldahl nitrogen determination, the final product is a solution of NH4 in HCl solution. It is necessary to titrate the HCl without titrating NH4. a. Calculate the pH of pure 0.010 M NH4Cl. b....
-
As societys cost of disposing of trash increases over time, recycling rates should automatically increase as well. Discuss.
-
Carol and Sam Foyle own Campus Fashions. From its inception Campus Fashions has sold merchandise on either a cash or credit basis, but no credit cards have been accepted. During the past several...
-
Analysis of the accounts of Jonimatt State College for the fiscal year ended June 30, 20X7, provided the following information: Revenues from: Tuition and fees... State appropriations .... Federal...
-
12-6. Cmo emplea un cine los precios fuera de las horas pico?
-
The so-called local carrier is also almost unique to the motor-carrier industry. Why?
-
HOMEWORK 06 15 POINTS The management of ABC company are considering buying a new machine which would produce parts for a client. They have 2 options: Machine A costs $65,000 (useful life: 7 years,...
-
Financial Statements of Peter Ltd. and its 80%-owned subsidiary Sam Ltd. as at December 31, Year 5, are presented below: Statement of Financial Position as at December 31 Year 5 Peter Ltd Sam Ltd...
-
Method of continuous variation. Make a graph of absorbance versus mole fraction of thiocyanate from the data in the table. (a) What is the stoichiometry of the predominant Fe(SCN) n 3-n species? (b)...
-
Simulating a Job's plot. Consider the reaction , for which K [AB 2 ]/[A][B ]2 . Suppose that the following mixtures of A and B at a fixed total concentration of 104M are prepared: (a) Prepare a...
-
1. Do you think the court made the right decision in this case? Explain. 2. Given that there is a statutory duty to bargain in good faith, why do you think management chose to do what it did? 3....
-
You want to retire after working 35 years with savings in excess of $1,100,000. You expect to save $3,300 a year for 35 years and earn an annual rate of Interest of 11%. (Round your answer to 2...
-
FOLLOW ALL INSTRUCTIONS AND GENERATE YOUR CODE AFTER READING THE JUNIT TESTS, THAT IS ALL THE METHODS AND CONSTRUCTORS YOU USE SHOULD BE BASED ON THE JUNIT TESTS PROVIDED. I HAVE ATTATCHED THE JAVA...
-
Are some values in the class data grossly different from all the others? If so, check for errors in calculation or procedure that would allow to objectively eliminate the data. 2. Do the range values...
-
An aging analysis of Uli Limited's accounts receivable at December 3 1 , 2 0 2 4 and 2 0 2 3 , showed the following: Number of Days Outstanding Accounts Receivable Estimated Percentage Uncollectible...
-
(Linear momentum) Two jets of liquid, one with specific gravity 1.00 and the other with specific gravity 1.33, collide and form one homogeneous jet as shown in the figure below. Determine (a) the...
-
Why does it reduce a contractor's risk to begin construction only after receiving authorization from the owner?
-
Planning: Creating an Audience Profile; Collaboration: Team Projects. Compare the Facebook pages of three companies in the same industry. Analyze the content on all available tabs. What can you...
-
You perform a series of experiments for the reaction A -- B + C and find that the rate law has the form. Rate = k[A]x. Determine the value of x in each of the following cases: (a) There is no rate...
-
Consider the data presented in Exercise 14.20. (a) Determine whether the reaction is first order or second order. (b) What is the rate constant? (c) What is the half-life?
-
The gas-phase decomposition of NO2, 2 NO2(g) 2 NO(g) + O2(g), is studied at 383 oC, giving the following data: Time (s) [NO2] (M) 0.0........................0.100 5.0........................0.017...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...

Study smarter with the SolutionInn App


