NaHCO3 is decomposed by heat according to If you start with 100.0 g of NaHCO3 and collect
Question:
NaHCO3 is decomposed by heat according to
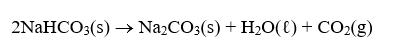 If you start with 100.0 g of NaHCO3 and collect 10.06 L of CO2 over water at 20°C and 0.977 atm, what is the percent yield of the decomposition reaction?
If you start with 100.0 g of NaHCO3 and collect 10.06 L of CO2 over water at 20°C and 0.977 atm, what is the percent yield of the decomposition reaction?
Transcribed Image Text:
2NAHCO3(s) → Na2CO3(s) + H20(t) + CO2(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
Here we have a stoichiometry problem where we need to find the amount of CO 2 collected when 100...View the full answer

Answered By

John Aketch
I am a dedicated person with high degree of professionalism, particularly in academic writing. My desire is to is to make students excel in their academic endeavor.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
NaHCO3 decomposes when exposed to heat: 2NaHCO3(s) ( Na2CO3(s) + CO2(g) + H2O() H = 91.5 kJ What mass of NaHCO3 is decomposed by 256 kJ?
-
What is the equilibrium composition of a reaction mixture if you start with 0.500 mol each of H2 and I2 in a 1.0-L vessel? The reaction is H2(g) + 12(g)--2HI(g) Kc = 49.7 at 458C
-
Part 1: You run the chemical reaction a. Write the equilibrium-constant expression for the reaction. b. Can you come up with some possible concentrations of C, D, and E that you might observe when...
-
How do complementary assets and social complexity influence a firm's organization?
-
How do the auditors evaluate misstatements in a substantive test?
-
In one of Thomsons experiments he placed a thin metal foil in the electron beam and measured its temperature rise. Consider a cathode-ray tube in which electrons are accelerated through a 2000 V...
-
Think about the last time you wrote a paper or report for school. Consider how to create standards, how to measure your performance, how to compare your performance to standards, and how to take...
-
1. Why would Nortel Networks, a Canadian company, hire a U.S. law firm to undertake an independent review of factors that led to restatement of accounting reports? 2. Why did the independent review...
-
**Step 1: Recognition of Share-Based Payments** In accounting for share-based payments under IFRS 2, the first step is the recognition of these payments in the financial statements. Share-based...
-
Match the following and select the correct option. Height of Binary search tree(in worst o([log ]-1) 1 a (2n+1) case) Height of Ternary tree b O(n) Height of B-tree(when c minimum degree, -2) (log,...
-
HNO3 reacts with iron metal according to Fe(s) + 2HNO3(aq) ( Fe(NO3)2(aq) + H2(g) In a reaction vessel, 23.8 g of Fe are reacted but only 446 mL of H2 are collected over water at 25C and a pressure...
-
What is the pressure in pascals if a force of 3.44104 MN is pressed against an area of 1.09 km2?
-
Solve the differential equation using the method of variation of parameters. y'' - 2y' + y = e X /1 + x 2
-
Share your thoughts on the descriptions of coaching versus mentoring. Discuss which technique you personally find more helpful, incorporating your peers' example scenarios if possible. Provide...
-
Hanung Corp has two service departments, Maintenance and Personnel. Maintenance Department costs of $380,000 are allocated on the basis of budgeted maintenance-hours. Personnel Department costs of...
-
Discuss difference between nominal interest rate and real interest rate. Explain why real interest rate is more important than the nominal interest rate using your answer to Question 1 of the...
-
Refer to Figure 14-1. How would an increase in the money supply move the economy in the short and long run?
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
Approximate the sum of the areas of the rectangles shown in Figure (18.8 b). That is, using \[A_{1}+A_{2}+\cdots+A_{15}+A_{16}\] Figure (18.8 b) 1.2 1.0 0.8 0.6 0.4 0.2 y y=x (1,1) 0.2 0.4 0.6 0.8...
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
A specific consumer group to which a product is designed to appeal Match the terms with the definitions. Some terms may not be used. a. company advertising b. custom c. economy d. industry...
-
The metallic bonding found in the element gold is such that 1.00 ounce of Au can be flattened into a sheet that is 3.00 10 2 ft 2 . If there are 28.35 g/ounce and 30.48 cm/ft, how thin is this...
-
What is the volume change when 1 mol of graphite (d = 2.26 g/cm 3 ) is converted to diamond (d = 3.51 g/cm 3 )?
-
Why do free-floating liquids in an orbiting spacecraft adopt a spherical shape?
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...

Study smarter with the SolutionInn App


