Name the following alkenes, and predict the products of their reaction with (1) meta-chloroperoxybenzoic acid, (ii) KMnO4
Question:
Name the following alkenes, and predict the products of their reaction with (1) meta-chloroperoxybenzoic acid, (ii) KMnO4 in aqueous acid, and (iii) O3, followed by Zn in aceticacid:
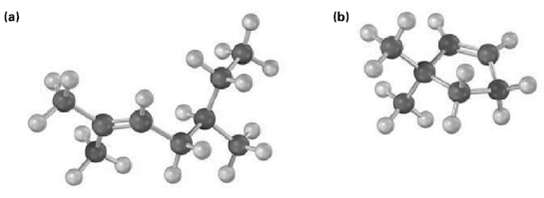
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
a CH3 CH3CCHCHCHCHCH3 25Dimethyl2heptene CH3 RCO3H KM...View the full answer

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Name the following alkynes, and predict the products of their reaction with (i) H2 in the presence of a Lindlar catalyst and (ii) H3O+ in the presence ofHgSO4: (b) (a)
-
Predict the products formed by periodic acid cleavage of the following diols. (a) CH3CH(OH)CH(OH)CH3 (b) (c) (d) CHAOH OH OH Ph-CCH OH)CH,CH CH HO HO
-
Name each of the following alkenes or alkynes. a. CH2 = CH-CH2-CH3 b. c. d. e. f. g. CH3 C-CH-CH3 CH3 CH CH3 CH3CH2CH CH CH CH CH3 CH, C-CH-CH CH, CH2-CH, CH3 CH2CHs CH, CH2CH3 CH3 C C-CH CH3 CH3
-
Consider a process consisting of five resources that are operated eight hours per day. The process works on three different products, A, B, and C; Resource Number of Workers Processing Time for A...
-
Sarpedon Corp. claims that its car batteries average at least 880 CCA (cold-cranking amps). Tests on a sample of 9 batteries yield a mean of 871 CCA with a standard deviation of 15.6 CCA. (a) State...
-
Comment on the market performance of companies going public, both immediately after the offering has been made and some time later. Relate this to research that has been done in this area.
-
22. Describe the circumstances in which an employee may not value a nontaxable fringe benefit.
-
Specialty Appliances and More, Inc. ( SAM) has a three- year warranty on their solar refrigerators for defects. Warranty costs are estimated at 2% of sales in year one ( the year of the sale), and 5%...
-
Statement of Cash Flows. W. C. Cycling had $ 55,000 in cash at year-end 2013 and $ 25,000 in cash at year-end 2014. The firm invested in property, plant, and equipment totaling $ 250,000. Cash flow...
-
The following data relate to the operations of Shilow Company, a wholesale distributor of consumer goods: Current assets as of March 31: 7,400 19,600 Cash Accounts receivable Inventory Building and...
-
One of the chain-termination steps that sometimes occurs to interrupt polymerization is the following reaction between two radicals. Propose a mechanism for the reaction, using fishhook arrows to...
-
Draw the structures of alkenes that would yield the following alcohols on hydration (red = O). Tell in each case whether you would use hydroboration/oxidation oroxymercuration. (b) (a)
-
Determine the force acting along the axis of each of the three struts needed to support the 500-kgblock. 25 m 125 m 3m 0.75 m
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 90% confident that the estimated percentage is in error...
-
Palmerstown Company established a subsidiary in a foreign country on January 1, Year 1, by investing 8,000,000 pounds when the exchange rate was $1.00/pound. Palmerstown negotiated a bank loan of...
-
Question 1.Which of the following plans provide the greatest immediate tax benefit for the participating employee? (1) Roth IRA (2) deductible IRA (3) non-deductible IRA (4) 401(k) a. (1) and (3)...
-
Transcribed image text: 9:13 LTE Done 7 of 7 QUESTION WA AUDION QUESTION 23 = w the tons of a coin comes down heads, you win two dollars. If it comes down tails, you lose fifty cents. How much would...
-
TRUE or FALSE It is 2016 and the D.C. Circuit has issued its ruling in USTA v. FCC . The D.C. Circuit upheld the 2015 Open Internet Order so the FCC's net neutrality rule stands.True or...
-
Discuss how one might draw a distinction between physical and dollar reports in terms of reports more related to control as such vs. reports related to reviewing the results of control. LO.1
-
A red card is illuminated by red light. What color will the card appear? What if its illuminated by blue light?
-
What happens to the vapor pressure of a substance when its surface area is increased at constant temperature? (a) The vapor pressure increases. (b) The vapor pressure remains the same. (c) The vapor...
-
A student, Flick Flaskflinger, in his twelfth year of graduate work, needed to prepare ethylmagnesium brdmide from ethyl bromide and magnesium, but found that his laboratory was out of diethyl ether....
-
When sec-butylbenzene undergoes free-radical bromi-nation, one major product is formed, If the starting material is optically active, predict whether the substitution product should also be optically...
-
Three alkyl halides, each with the formula C7HBr, have different boiling points. One of the compounds is optically active. Following reaction with Mg in ether, then with water, each compound gives...
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...

Study smarter with the SolutionInn App


