Name the following alkenes, including the cis or Tran?s designation: (b)
Question:
Name the following alkenes, including the cis or Tran?s designation:
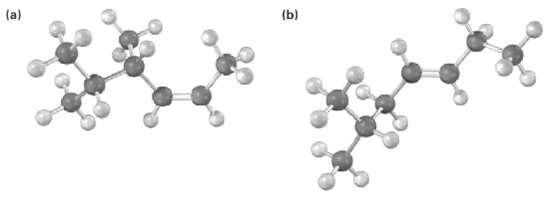
Transcribed Image Text:
(b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
H3 CH3 CH3CHCH CH...View the full answer

Answered By

Leah Muchiri
I am graduate in Bachelor of Actuarial Science and a certified accountant. I am also a prolific writer with six years experience in academic writing. My working principle are being timely and delivering 100% plagiarized free work. I usually present a precised solution to every work am assigned to do. Most of my student earn A++ GRADE using my precised and correct solutions.
4.90+
52+ Reviews
125+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Name each of the following alkenes or alkynes. a. CH2 = CH-CH2-CH3 b. c. d. e. f. g. CH3 C-CH-CH3 CH3 CH CH3 CH3CH2CH CH CH CH CH3 CH, C-CH-CH CH, CH2-CH, CH3 CH2CHs CH, CH2CH3 CH3 C C-CH CH3 CH3
-
Name each of the compounds below. Use cis / trans and/or E/Z designations, if appropriate, to designate stereochemistry a. b. c. Cl C=C H,C CH3 CH2CI H3C H3C H,C Cl
-
The name Computer Emergency Response Team is the historic designation for the first team (CERT/CC) at Carnegie Mellon University (CMU). CERT is now a registered service mark of Carnegie Mellon...
-
Suppose the money supply is $250 million dollars and the demand for money is given by Qm D = 400 - 40i, where Qm D is in millions of dollars. A. What is the equilibrium interest rate in this economy?...
-
Which of the four path-goal styles do you think would be the best for managing a group of software engineers? Justify your answer.
-
(a) A point charge Q lies at the origin. Show that div D is zero everywhere except at the origin. (b) Replace the point charge with a uniform volume charge density v0 for 0 < r < a. Relate v0 to Q...
-
How can management use the DuPont equation to analyze ways of improving the firms performance? AppendixLO1
-
Prior to adjustment at the end of the year, the balance in Trucks is $250,900 and the balance in Accumulated Depreciation?Trucks is $88,200. Details of the subsidiary ledger are as follows: (a)...
-
2. Use a graph of the pollution abatement market (i.e., MSC and MSB of abatement) to model each of the following situations: a. A market where the allocatively efficient level of abatement occurs at...
-
All businesses are involved in three types of activitiesfinancing, investing, and operating. Listed below are the names and descriptions of companies in several different industries. Abitibi...
-
Which of the following compounds can exist as pairs of cisTrans isomers? Draw each cisTrans pair, and indicate the geometry of each isomer. (a) CH3CH = CH2 (b) (CH3)2C = CHCH3 (c) CH3CH2CH = CHCH3...
-
Which member in each of the following sets has higher priority? (a) H or Br (b) C1 or Br (c) CH 3 or CH 2 CH 3 (d) NH 2 or OH (e) CH 2 OH or CH 3 (f) CH 2 OH or CH = O
-
Joan died April 17, 2017. Joan's executor chose March 31 as the tax year end for the estate. The estate's only beneficiary, Kathy, reports on a calendar year. The executor of Joan's estate makes the...
-
You have been employed as a systems analyst in the information systems organization of a medium-sized consumer goods manufacturer for three years. You are quite surprised when your manager offers you...
-
For your initial post, address the following: First, introduce yourself to the class by sharing a bit about yourself, such as your preferred name or pronouns, where you are from, what your major is,...
-
Question 8 : Consider the technology of Solar Panels. Which stage of the technology life cycle S curve is this technology in. Justify why ? Question 9 : The standard Product Life Cycle has 5 stages...
-
At Benihana restaurant a man wrenched his neck while ducking a piece of flying shrimp, requiring treatment by several doctors. By that summer, doctors determined surgery was necessary to treat...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
If Stephen continues to refuse to relinquish the tip, what steps, if any, can management take to force him to do so?
-
Wimot Trucking Corporation uses the units-of-production depreciation method because units-of-production best measures wear and tear on the trucks. Consider these facts about one Mack truck in the...
-
Nitrogen has a normal boiling point of 77.3 K and a melting point (at 1 atm) of 63.1 K. Its critical temperature is 126.2 K, and its critical pressure is 2.55 * 10 4 torr. It has a triple point at...
-
Draw the structure of 4-isopropyl-2,4,5-trimethylheptane.
-
What is the dissociation constant of an acid that has a pKa of (a) 4 (b) 7.8 (c) -2
-
Using the pka values in Table 3.1, calculate the equilibrium constant for the following reaction. F- acting as a base toward the acid HCN
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


