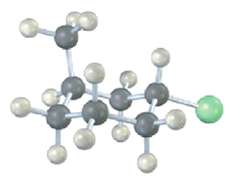
Name the following compound, identify each substituent as axial or equatorial, and tell whether the conformation shown
Question:
Name the following compound, identify each substituent as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (yellow-greenC1):
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
aCH3 H H CCI ring flip HCe H H tra...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The most stable conformation of 1,3-dioxan-5-ol is the chair form that has its hydroxyl group in an axial orientation. Suggest a reasonable explanation for this fact. Building a molecular model is...
-
Identify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (yellow green ? CI):
-
The most stable conformation of trqans-l,2-cyclohexanediol is the chair in which both hydroxy groups are equatorial, (a) Draw the structure or, better yet, make a model of the compound in this...
-
Why does allocating an array of length \(n\) take time proportional to \(n\) ?
-
Assume you purchased a high-yield corporate bond at its current market price of $850 on January 2, 2004. It pays 9 percent interest and it will mature on December 31, 2013, at which time the...
-
Explain how unethical marketers might use bait-and-switch tactics, price-fixing, and predatory pricing. What is surge pricing?
-
What is direct posting of sales invoices? AppendixLO1
-
The total factory overhead for Klein Calvin is budgeted for the year at $ 225,000, divided into four activities: cutting, $ 22,500; sewing, $ 45,000; setup, $ 100,000; and inspection, $ 57,500. Klein...
-
What is meant by a hard market and a soft market within the insurance industry?
-
Find the inductance of the cone-sphere configuration described in Problem 8.35 and Figure 8.17. The inductance is that offered at the origin between the vertices of the cone. Figure 8.17 21 r= 0.25 m...
-
Name the following cycloalkanes: (a) (b)
-
A trisubstituted cyclohexane with three substituents? red, yellow, and blue?undergoes a ring-flip to its alternative chair conformation. Identify each substituent as axial or equatorial, and show the...
-
On January 1, 2015, Encino Company issued bonds with a face value of $1,000,000 and a maturity date of December 31, 2024. The bonds have a stated interest rate of 8%, payable on January 1 and July 1....
-
I have attached a case study, primarily based on your textbook chapter reading assignments. The background material for the case also references chapters 3 and 15, not assigned for this course....
-
On December 1 , 2 0 2 5 , Sandhill Distributing Company had the following account balances.DebitCash$ 7 , 1 0 0 Accounts Receivable 4 , 5 0 0 Inventory 1 1 , 9 0 0 Supplies 1 , 2 0 0 Equipment 2 2 ,...
-
Cindy Greene works at Georgia Mountain Hospital. The hospital experiences a lot of business closer to summer when the temperature is warmer. Cindy is meeting with her supervisor to go over the budget...
-
Use z scores to compare the given values. Based on sample data, newborn males have weights with a mean of 3247.4 g and a standard deviation of 575.4 g. Newborn females have weights with a mean of...
-
Gignment FULL SCAL Exercise 4- The following ndependent situations require professional judgment for determining when to recognize revenue from the transactions. Identify when revenue should be...
-
One of the following statements about the sole trader form of business is correct: a) A sole trader has to pay corporation tax. b) Sole traders submit annual information to the Registrar of...
-
From 1970 to 1990, Sri Lanka's population grew by approximately 2.2 million persons every five years. The population in 1970 was 12.2 million people.What is the best formula for P, Sri Lanka's...
-
Butanol and pentane have approximately the same mass, however, the viscosity (at 20 C) of butanol is = 2.948 cP, and the viscosity of pentane is cP. Explain this difference.
-
When the 3-bromo-2-butanol with the stereochemical structure A is treated with concentrated HBr, it yields meso-2,3-dibromobutane; a similar reaction of the 3-bromo-2-butanol B yields...
-
Reaction of an alcohol with thionyl chloride in the presence of a tertiary amine (e.g., pyridine) affords replacement of the OH group by Cl with inversion of configuration (Section 11.9). However, if...
-
Draw all of the stereoisomers that are possible for 1,2,3-cyclopentanetriol. Label their chirality centers and say which are enantiomers and which are diastereomers. (Some of the isomers contain a...
-
Current Attempt in Progress Assume that on January 1 , 2 0 2 5 , Sheffield Corporation sells equipment to Sheridan Finance Co . for $ 1 , 8 2 0 , 0 0 0 and immediately leases back the equipment. The...
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.

Study smarter with the SolutionInn App


