Name the following compounds: (b)
Question:
Name the following compounds:
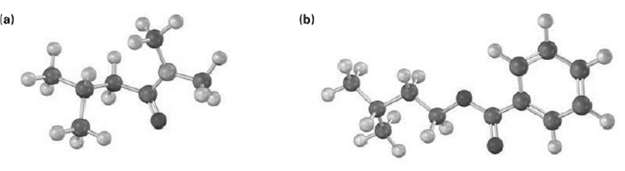
Transcribed Image Text:
(b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a H3C H3CH CH3 CH...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Name the following compounds by IUPAC rules: a. b. H-C CH,CH-CH
-
Name the following compounds by the IUPAC system: a. CH3CH=C(CH2CH2CH3)2 b. (CH3)2CHCH"CHCH3 c. g. CH3-C-C-CH-CH, h. k.
-
Name the following compounds and assign oxidation states to the halogens in them: (a) Fe(ClO3)3 (b) HClO2 (c) XeF6 (d) BrF5 (e) XeOF4 (f) HIO3.
-
Researchers examined forecasters' interest rate predictions for 34 quarters to see whether the predictions corresponded to what actually happened. The 2 x 2 contingency table below shows the...
-
Why is redundancy-usually condemned in business communication-a smart strategy in organizing the main points in the introduction, body, and conclusion of your presentation?
-
A rectangular coil is composed of 150 turns of a filamentary conductor. Find the mutual inductance in free space between this coil and an infinite straight filament on the z axis if the four corners...
-
Executive salaries have been shown to be more closely correlated to the size of the firm than to its profitability. If a firms board of directors is controlled by management rather than outside...
-
Two point charges, Q1 = - 25μC and Q2 = + 50μC, are separated by a distance of 12cm. The electric field at the point P (See Fig. 16-55) is zero. How far from Q1 is P? 12 cm -25 Q2 P. +50
-
Icebreaker Company (a U.S.-based company) sells parts to a foreign customer on December 1, 2020, with payment of 34,000 dinars to be received on March 1, 2021. Icebreaker enters into a forward...
-
1. What are the strengths and weaknesses of the Campbell Soup Company's marketing information system? 2. What objectives does Campbell have for the marketing research efforts described in this case?...
-
Propose structures for compounds that have the following formulas and IR absorptions: (a) C6H12O2, 1735 cm1 (b) C4H9NO, 1650 cm1 (c) C4H5ClO, 1780 cm1
-
How would you prepare the following compounds starting with an appropriate carboxylic acid and any other reagents needed? (Reddish brown =Br.) (a) (b)
-
A liquid mixture containing 40% cyclohexane, 20% benzene, 25% toluene, and 15% n-heptane is in equilibrium with its vapor at 1 bar. Determine the temperature and the vapor composition.
-
What should be the equivalent units of production for (1) Dept M and (2) Dept. P? Can you please show the solutions and answer. Thanks Problem 1 Lee Gon Mfg. Co has its product processed in two...
-
Moullierat Mfg. is considering a rights offer. The company has determined that the ex-rights price will be $95. The current price is $102 per share, and there are 24 million shares outstanding. The...
-
This question involves hypothesis testing. The following numbers will help you answer these questions. The random variable Z ~N(0, 1) is standard normal. P(Z >1.28).1 P(Z1.65) .05 P(Z1.96) .025 P(Z...
-
Human service organizations require strong and effective leadership. Understanding what qualities make up an effective leader and how these qualities can be cultivated is of critical importance for...
-
18. What is the name of the heat treatment performed on a cold worked sample? 19. What is the percent coldwork of a sample with an initial thickness of 11mm and a final thickness of 7mm? 20. Which...
-
Utilize alternative methods when establishing a hotels room rate structure.
-
Pedro Bourbone is the founder and owner of a highly successful small business and, over the past several years, has accumulated a significant amount of personal wealth. His portfolio of stocks and...
-
For each compound, draw the Lewis structure, determine the geometry using VSEPR theory, determine whether the molecule is polar, identify the hybridization of all interior atoms, and make a sketch of...
-
The proton-decoupled 13C NMR spectra of 3-heptanol (A) and 4-heptanol (B) are given in Fig. 13.22 on page 626. Indicate which compound goes with each spectrum, and explain your reasoning. Fig. 13.22...
-
The proton-decoupled 13C NMR spectra of 3-heptanol (A) and 4-heptanol (B) are given in Fig. 13.22 on page 626. Indicate which compound goes with each spectrum, and explain your reasoning. Fig. 13.22...
-
Explain why each of the following structures is not consistent with the 13C NMR data in Study Problem 13.6. Problem 13.6 TABLE 13.1 Effect of Electronegativity on Proton Chemical Shift Chemical...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


