On reaction with acid, 4-pyrone is protonated on the carbonyl-group oxygen to give a stable cationic product.
Question:
On reaction with acid, 4-pyrone is protonated on the carbonyl-group oxygen to give a stable cationic product. Using resonance structures and the Hückel 4n + 2 rule, explain why the protonated product is sostable.
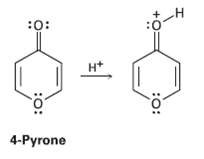
Transcribed Image Text:
:0: :0: H* 4-Pyrone :o: :o:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
666666 Protonation of 4pyrone gives structure A whic...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the structures of the carbonyl compound and the amine used to form the following imines. (a) (b) (c) (d) (e) (f) NH N=CHCH, N CH
-
Give the product of the reaction of pentanoic acid with each of the following reagents: (a) Sodium hydroxide (b) Sodium bicarbonate (c) Thionyl chloride (d) Phosphorus tribromide (e) Benzyl alcohol,...
-
Give an example of (a) A weak acid that contains oxygen atoms. (b) A weak acid that does not contain oxygen atoms. (c) A neutral molecule that acts as a Lewis acid. (d) A neutral molecule that acts...
-
Refer to the Journal of Experimental Psychology-Applied (June 2000) name-retrieval study, presented in Exercise. The data for the study are saved in the NAMEGAME2 file. a. Find a 99% confidence...
-
What place would efficient job design have in a company like BraunAbility? How could BraunAbility improve job efficiency in a way that is consistent with the company's emphasis on inclusiveness and...
-
Carrington Oil produces gas 1 and gas 2 from two types of crude oil: crude 1 and crude 2. Gas 1 is allowed to contain up to 4% impurities, and gas 2 is allowed to contain up to 3% impurities. Gas 1...
-
Contact your local police department or state police. Identify the BAL in your state in which a driver is considered legally intoxicated. Discover whether the same BAL applies to minors. Ask for...
-
Scotch whisky increases in value as it ages, at least up to a point. For any period of time, t, the value of a barrel is given by V = 100t 6t2. This function implies that the proportional rate of...
-
1. Why do we bother with break-even analysis? A business is formed to earn profit, not to just make a loss( e.g. break-even). Discuss. 2 Would you expect the operating profit margin to be higher or...
-
Maria A Solo (SSN 318-01-6921) lives at 190 Glenn drive, grand rapids, Michigan 49527-2005. Maria (age 45 and single) claims her aunt, Selda Ray (ssn 282-61-4011), as a dependent. Selda lives with...
-
Bextra, a COX-2 inhibitor used in the treatment of arthritis, contains an isoxazole ring. Why is the ringaromatic? Isoxazole ring H2N CH3 Bextra
-
Compound A, C8H10, yields three substitution products, C8H9Br, on reaction with Br2. Propose two possible structures for A. The 1H NMR spectrum of A shows a complex four-proton multiplet at 7.0 and...
-
Why does net working capital appear with both negative and positive values in Figure 13-2? FIGURE 13-2 Analysis of an Expansion Project: Cash Flows and Performance Measures (Thousands of Dollars) B C...
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
In Exercises 13 and 14, use the box-and-whisker plot to identify the five-number summary. 0 2 5 8 10 ++ ++ 0 1 2 3 4 5 6 7 8 9 10 11
-
In a test of the effect of dampness on electrical connections, 80 electrical connections were tested under damp conditions and 130 were tested under dry conditions. Twenty of the damp connections...
-
Zelta Ltd. is a medium-size company involved in providing a range of specialized products and services for the aerospace industry. Just over a year ago, external consultants undertook a major review...
-
explain the two main methods of branch accounting;
-
What are bounds and what do companies do with them?
-
Express the equilibrium constant for the chemical equation: CH3OH(g) CO(g) + 2 H(8)
-
(a) Circle the isoprene units in the following terpenes. (b) Classify each of these as a monoterpene, diterpene, etc. -farnese ne (from oil of citronella limonene -plnene zingiberene (from ol of...
-
Draw the structure of an example of each of the following types of lipids: (a) A saturated fat (b) A polyunsaturated oil (c) A wax (d) A soap (e) A detergent (f) A phospholipid (g) A prostaglandin...
-
Give the general classification of each compound. (a) glyceryl tripalmitate (b) (c) (d) (e) (f) CH3(CH210 CH2 Na CH3 (CH21C (CH216-CH3 tetradecyl octadecanoate CH3 CH HoC caryophyllene (from cloves)...
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...
-
A section 83(b) election creates ordinary income at the time of the grant. Ture or False

Study smarter with the SolutionInn App


