Question: One mole of an ideal monatomic gas at an initial volume V1 = 25 L follows the cycle shown in figure. All the processes are
One mole of an ideal monatomic gas at an initial volume V1 = 25 L follows the cycle shown in figure. All the processes are quasi-static. Find
(a) The temperature of each state of the cycle,
(b) The heat flow for each part of the cycle, and
(c) The efficiency of thecycle.
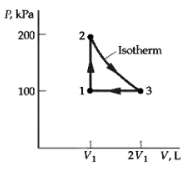
P, kP | 200 2. Isotherm 100 2V1 V,L V1
Step by Step Solution
★★★★★
3.50 Rating (167 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

a Using PV nRT T 1 100 258314 K 3007 K T 2 T 3 6014 K b Q 1... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
10-P-T-S-L (286).docx
120 KBs Word File


