One of the most historically significant studies of chemical reaction rates was that by M. Bodenstein (z.
Question:
One of the most historically significant studies of chemical reaction rates was that by M. Bodenstein (z. physik. Chem. 29,295 (1899)) of the gas-phase reaction 2 Hl(g) -t H2(g) + I2(g) and its reverse, with rate constants k and k', respectively. The measured rate constants as a function of temperature are
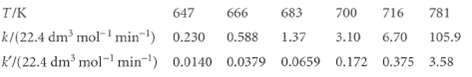
Demonstrate that these data are consistent with the collision theory of bimolecular gas-phase reactions.
Transcribed Image Text:
T/K 647 666 683 700 716 781 k/(22.4 dm³ mol ¹ min¹) 0.230 0.588 1.37 3.10 6.70 105.9 K/(22.4 dm³ mol-¹ min¹) 0.0140 0.0379 0.0659 0.172 0.375 3.58
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
Linear regression analysis of Inrate constant against 1T yields the following results In k224 dm mol ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
One of the most common conflicts in an organization occurs with raw materials and finished goods. Why would finance/accounting, marketing/sales, and manufacturing have disagreements?
-
The prisoners dilemma game is one of the most important models in all of social science: Most games of trust can be thought of as some kind of prisoners dilemma. Heres the classic game: Two men rob a...
-
The guanidino group of arginine is one of the most strongly basic of all organic groups. Explain. NH NI NI
-
Write a method to take an integer array as a parameter and return how many elements having the same digits in each element in the array for example if we have array 1 1 , 4 4 , 1 4 , 2 3 , 1 2 , 5 6...
-
A cylindrical piece of steel 50 mm (2 in.) in diameter is to be quenched in moderately agitated water. Surface and center hard-nesses must be at least 50 and 40 HRC, respectively. Which of the...
-
What are two operational reports produced by the FRS that provide proof to the accuracy of the process?
-
3. On the books of Pam Corporation, the investment account is properly reflected on December 31, 2016, at: a $3,240 b $3,264 c $3,276 d Not enough information is given.
-
Glaus Leasing Company agrees to lease equipment to Jensen Corporation on January 1, 2020. The following information relates to the lease agreement. 1. The term of the lease is 7 years with no renewal...
-
Question 3 Marathon Goods Ltd is a wholesale distribution firm. The following information relates to the movement in stores of its key product - XK . Sales of 1 , 0 0 0 units @ 2 0 per item are...
-
Wedding Planners Limited (WP), owned by Anne and Francois Tremblay, provides wedding planning and related services. WP owns a building (the Pavilion) that has been custom-made for hosting weddings....
-
For the thermal decomposition Of F2O by the reaction 2 F2O (g) 2 Fl (g) + 0z (g),) Czarnowski and H) Schumacher (Chem. Phys. Lett. 17, 235 (1972)) have suggested the following mechanism: (a) Using...
-
(a) Distinguish between a step and a terrace. (b) Describe how steps and terraces can be formed by dislocations.
-
There are 10 qualified applicants for 3 security positions at a hospital. In how many ways can the positions be filled?
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
Distinguish between: (i) Funds Flow Statement and Schedule of Changes in working capital. (ii) Net profit and Funds from operations.
-
Based on the scenario described below, generate all possible association rules with values for confidence, support (for dependent), and lift. Submit your solutions in a Word document (name it...
-
Find the area of the shaded region. y y= x+2 y = x= x = 2 1 x+1 X
-
(a) By considering the dependence of the Gibbs free energy of reaction on potential and on temperature, derive an equation for the temperature dependence of E cell . (b) Use your equation to predict...
-
(a) What is the standard cell potential (E cell ) for the reaction below at 298 K? (b) What is the standard cell potential for the reaction at 335 K? (c) What is the cell potential for the reaction...
-
The following items are obtained from a stockroom for the construction of a galvanic cell: two 250-mL beakers and a salt bridge, a voltmeter with attached wires and clips, 200 mL of 0.0080 m CrCl 3...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...

Study smarter with the SolutionInn App


