Predict the approximate chemical shifts of the absorptions in the 13C-NMR spectrum of thiscompound: CH,CH3 CH CH3
Question:
Predict the approximate chemical shifts of the absorptions in the 13C-NMR spectrum of thiscompound:
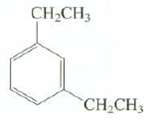
Transcribed Image Text:
CH,CH3 CH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 43% (16 reviews)
CHCH3 ...View the full answer

Answered By

Tamil Elakkiya Rajendran
I'm currently involved in the research in the field of Biothermodynamics, Metabolic pathway analysis and computational Biology. I always prefer to share my knowledge whatever I have learnt through my degree whenever time permits.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the approximate chemical shifts of the protons in the following compounds. (a) Benzene (b) Cyclohexane (c) CH3-O-CH2CH2CH2Cl (d) CH3CH2-C¡¡C-H (e) (f) (CH3)2CH-O-CH2CH2OH (g) (h)...
-
3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 ?. Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be...
-
Predict the approximate chemical shifts for the different hydrogen's in thesecompounds: CI CI a) CH,CH,CH3 b) CH;CHCH3 c) CH,COCH,CH3 d) CH;CHCH2
-
Which of the following is true about a statement of cash flows? The statement of cash flows is prepared at the option of management. The statement of cash flows is required by generally-accepted...
-
In note 4.6, AF indicates that "Both passenger and cargo tickets are recorded as "Deferred revenue on ticket sales" and that "Sales related to air transportation are recognized when the...
-
The tangent line to the graph of f(x) at x = 1 is shown. On the tangent line, P is the point of tangency and A is another point on the line. (a) Find the coordinates of the points P and A. (b) Use...
-
Refer to the Journal of Genetic Psychology (Mar. 1998) comparison of male and female college students' attitudes toward their fathers, Exercise 7.10 (p. 357). In this exercise, data adapted from the...
-
Ronald Wayne Smith was employed by Modesto High School as a temporary math instructor. In addition, he coached the girls baseball and basketball teams. The contract under which he was employed stated...
-
How to calculate Macaulys Duration Please provide formula and example
-
Shown below is a segmented income statement for Hickory Companys three wooden flooring product lines: Hickorys parquet flooring product line has a contribution margin of $50,000 (sales of $300,000...
-
Predict the approximate chemical shifts, multiplicities and integrals for the absorptions in the 1H-NMR spectra of thesecompounds: CH2 b) .- COCH CH3 a) d) CH;CHCH CH3 c) CH;CH,NHCH,CH3
-
Suggest how these compounds could be distinguished by using NMRspectroscopy: CH CCH3 C-CH a) and CI CI CI b) and CI c) and H. d) and
-
Map what you think are the different expressions of the individual and teams levels in Omega.
-
Solve (c) 8 WI n=1 5 cos n N5
-
- Pierce Company reported net income of $160,000 with income tax expense of $19,000 for 2020. Depreciation recorded on buildings and equipment amounted to $80,000 for the year. Balances of the...
-
ABC Company had the following results as of 12/31/2020: ABC's hurdle rate is 10% CONTROLLABLE REVENUE CONTROLLABLE COST CONTROLLABLE ASSETS CONTROLLABLE INCOME 21. What is the division's margin? A....
-
A gray kangaroo can bound across a flat stretch of ground with each jump carrying it 10 m from the takeoff point. If the kangaroo leaves the ground at a 20 angle, what are its (a) takeoff speed and...
-
Since 1900, many new theories in physics have changed the way that physicists view the world. Create a presentation that will explain to middle school students why Quantum Mechanics is important, how...
-
Discuss the requirements of proximity, foreseeability and reasonability and applying the facts of this case, establish why a duty of care is owed to the claimant. Explain the legal basis for your...
-
Provide a draft/outline of legal research involving an indigenous Canadian woman charged with assault causing bodily harm under (Sec 267b) of the Criminal Code, where the crown wants a 12-month jail...
-
Getting a business off the ground requires more than a lot of hard work. It requires raising hard cash. Many small businesses have trouble raising seed money to get started, but those that succeed in...
-
Vinyl-substituted Cyclopropane undergo thermal rearrangement to yield cyclopentane. Propose a mechanism for the reaction, and identify the pen- cyclic processinvolved. Heat Vinylcyclopropane...
-
The following reaction takes place in two steps, one of which is a cyclo addition and the other of which is a reverse cycloaddition. Identify the two pericyclic reactions, and show how theyoccur. ,H...
-
Two sequential pericyclic reactions are involved in the following furan synthesis. Identify them, and propose a mechanism for thetransformation. CH C. CH C6H5C=N at "
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


