Predict the product(s) of the following reactions: CH (b) CH-CH (a) 1. DIBAH 2. H30+ 1. CH2CH,MgBr
Question:
Predict the product(s) of the following reactions:
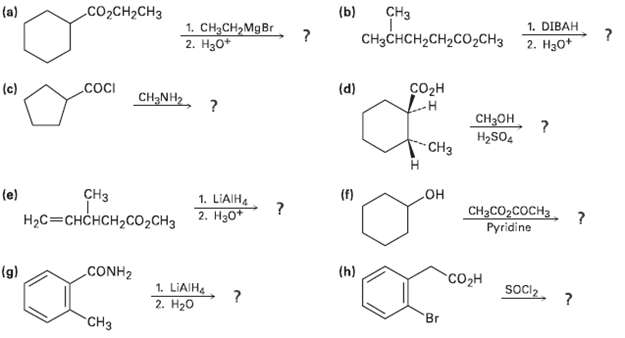
Transcribed Image Text:
CHз (b) СооCH-CHз (a) 1. DIBAH 2. H30+ 1. CH2CH,MgBr 2. Нао" CHзснCH-CH2CO2CH3 (d) Созн .cocI (c) CH NH2, ? CH3он H2SO4 "CHз но CH3CO2COCH3 (f) СНз 1. LIAIH (e) 2. Нзо" Pyridine НаС3 СHCHCH>со,сна (h) "сон CONH2 (g) Socl2. 1. LIAIH. 2. H20 Br "CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a b c O0 OCHCH3 1 CH3CHMgBr 2 H30 CH3 CH3CHCHCHCOCH 3 i The 1 DIBA...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the product of each of the following Diels-Alder reactions for the diene (left) and dienophile (right) as shown below. 0 0 0 0
-
Predict whether the following reactions will be spontaneous in acidic solution under standard conditions: (a) Oxidation of Sn to Sn2+ by I2 (to form I-) (b) Reduction of Ni2+ to Ni by I- (to form I2)...
-
Predict whether the following reactions would occur spontaneously in aqueous solution at 25C. Assume that the initial concentrations of dissolved species are all 1.0 M. (a) Ca(s) + Cd2+(aq) Ca2+(aq)...
-
A man drops a stone from a high bridge and hears it strike the water below exactly 4 s later. (a) Estimate the distance to the water based on the assumption that the travel time for the sound to...
-
Why should even practiced speakers plan their presentations when addressing a business audience instead of just "winging it"?
-
A 9.4 10 21 kg moon orbits a distant planet in a circular orbit of radius 1.5 10 8 m. It experiences a 1.1 10 19 N gravitational pull from the planet. What is the moons orbital period in earth...
-
What is treasury stock? AppendixLO1
-
Golden Corporation has $20,000,000 of 7 percent, 20-year bonds dated June 1, 2010, with interest payment dates of May 31 and November 30. After 10 years, the bonds are callable at 104, and each...
-
If the beginning balance of Accumulated DepreciationEquipment is $16,000 and an adjusting journal entry for depreciation on the equipment for $4,400 is omitted at the end of the period, Accumulated...
-
Let S= {a,b,c,d,e} and P be the set of partitions of S such that P={P1,P2,P3,P4}, where P1={{a,b,c},{d,e}},P2={{a,b},{c,d,e}},P3={{a,b,c,d,e}} and P4= {{a},{b},{c},{d},{e}} A partial order is defined...
-
How might you prepare the following compounds from butanoic acid? (a) 1-Bulanol (b) Butanal (c) 1-Bromobutane (d) Pentanenitrile (e) 1-Butene (f) N-Methylpentanamidc (g) 2-Hexanone (h) Butyl benzene...
-
Predict the product, if any, of reaction between propanoyl chloride and the following reagents: (a) Li (Ph) 2 Cu in ether (b) LiA1H 4 , then H 3 O + (c) CH 3 MgBr, then H 3 O + (d) H 3 O + (e)...
-
Which of the following strategies can be used to deal with missing values? a. Keep. b. Delete. c. Replace/impute. d. All of the above.
-
Write a java program that contain two overloaded methods that accepts two numbers or two characters representing a range example (11, 37) or (c, w) inputted by the user. The method generates a random...
-
Maggie could not conceive a child using natural means, so she sought out a woman who would donate an egg to be surgically implanted in Maggie. Which of the following items are deductible by Maggie in...
-
M corporation is subject to tax only in state b state b law provides for the use of federal taxable income before net operating loss and special deductions as the starting point for computing state...
-
Use Routh Criteria to determine the values of K needed for the system represented by the Characteristic Equation to be stable. (1 + K)s + (2K + 3)s + 2 3K = 0 Obtain the root locus plot for the...
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
Identify the aspects of an organizations environment that are most strategically important AppendixLO1
-
Modify the counter from Exercise 5.44 such that the counter will either increment by 4 or load a new 32-bit value, D, on each clock edge, depending on a control signal Load. When Load = 1, the...
-
The valence electron configurations of several atoms are shown here. How many bonds can each atom make without hybridization? a. Be 2s 2 b. P 3s 2 3p 3 c. F 2s 2 2p 5
-
The 60-MHz proton NMR spectrum of 2, 2, 3, 3-tetra-chlorobutane consists of a sharp singlet at 25oC, but at -45oC consists of two singlets of different intensities separated by about 10 Hz. Explain...
-
The 60-MHz proton NMR spectrum of 2, 2, 3, 3-tetra-chlorobutane consists of a sharp singlet at 25oC, but at -45oC consists of two singlets of different intensities separated by about 10 Hz. Explain...
-
What changes would you expect in the 13C NMR spectrum of 1-bromopropane upon cooling the compound to very low temperature?
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...
-
International business and environment The MIR requires teams to gather current, or the most recently available, data on the markets people, economy, government, and technological status from online...
-
Consider the following stream of cash flows. The interest rate is 10%. 0 1 2 3 4 5 6 7 100 100 100 200 0 300 300 300 a) What is the value at time 0 of the cash flow stream? b) What is the value of...

Study smarter with the SolutionInn App


