Predict the structure of the product formed in the reaction of the organic base pyridine with the
Question:
Predict the structure of the product formed in the reaction of the organic base pyridine with the organic acid acetic acid, and use curved arrows to indicate the direction of electronflow.
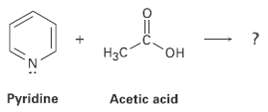
Transcribed Image Text:
Нас он Pyridine Acetic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
CN N...View the full answer

Answered By

David Ngaruiya
i am a smart worker who concentrates on the content according to my clients' specifications and requirements.
4.50+
7+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Use curved arrows to track electron movement in the dehydrohalogenation of tert-butyl chloride by sodium methoxide by the E2 mechanism.
-
Use curved arrows to show how calcium carbide reacts with water to give acetylene.
-
Use curved arrows to show the movement of electrons in each of the following reaction steps: a. b. c. d. Br O: " CH,COH + H-O-H CH,COH + H2O - CH3 CH3 CH CH3 CH
-
Describe four different definitions of quality.
-
Bernadette, a longtime client of yours, is an architect and the president of the local Rotary chapter. To keep up to date with the latest developments in her profession, she attends continuing...
-
Why does it seem to be necessary for the owner(s) to be on the premises all the time? Can there be personality cults in small businesses? Is it possible for customers to create a personality cult...
-
Under communism, there was limited freedom of choice. Do these countries remain less free in this sense than the countries of Western Europe? Use the WVS variable FREEDOM in a t-test to find out,...
-
How do you think each of the following items would affect a companys ability to attract new capital and the flotation costs involved in doing so? a. A decision of a privately held company to go...
-
Annas two production de them Doment predmetom had made the following state HOW 2. Machine-hours Det labe-hours Yotal fixed facturing over tout Varufacturing Variable manufacturing our per During the...
-
You recently graduated from college, and your job search led you to East Coast Yachts. Because you felt the companys business was seaworthy, you accepted a job offer. The first day on the job, while...
-
Is tert-butoxide anion a strong enough base to react with water? In other words, can a solution of potassium tert-butoxide be prepared in water? The pKa of tert-butyl alcohol is approximately18. CH...
-
Calculate Ka values from the following pKas: (a) Acetone, pKa = 19.3 (b) Formic acid, pKa = 3.75
-
Visithttp://totalqualitymanagement.wordpress.com/2008/10/28/lean-production-system, and compare the concepts of JIT/lean production system there with the information given in the chapter.
-
The composition of moist air is given on a molar basis to be 78 percent N2, 20 percent O2, and 2 percent water vapor. Determine the mass fractions of the constituents of air. Use the table containing...
-
1. Consider the LFSR with so = 1, 8 = 1, S2 = 1, 83 = 1, 84 = 0, and Sn Sn-2 Sn-3+ Sn-5. Find the next 15 terms in this LFSR. What is the period of this LFSR? 2. Suppose you learn that a Hill cipher...
-
Assume that you are thinking of a new acquisition campaign for SEDO, assuming that you want to convert people who are already engaged. Develop a big idea (in the communication) that you can use in...
-
You have a backend Amazon EC2 instance providing a web service to your web server instances. Your web servers are in a public subnet. You would like to block inbound requests from the internet to...
-
Consider the following task set. Task C T|D T1 20 50 40 T2 10 40 30 T3 5 20 15 a) Verify whether the task set is schedulable under DM using the processor utilization-based ap- proach. b) Verify...
-
Which statement is least appropriate? Audit management must avoid delays in releasing the draft audit report by using the following approach: a. Introduce technology to ensure reports can be...
-
(a) How far away can a human eye distinguish two ear headlights 2.0 m apart? Consider only diffraction effects and assume an eye pupil diameter of 5.0 mm and a wavelength of 550 nm. (b) What is the...
-
Redo Problem 4.45 if ethylene is described by the truncated virial equation with B (T) = 5.86 10 5 0.056/T m 3 /mol and T in K. Problem 4.45 The second virial coefficient B can be obtained from...
-
An unknown compound, X has the formula C6H12. (a) Calculate the degree of un-saturation of X. (b) X reacts with H2 in the presence of a catalyst to form a compound, Y, with the formula C6H14. What...
-
How many stereo isomers exist for this compound? Assign the relative stabilities of each. Is the methyl group axial or equatorial in the more stable conformer of the least stable stereo isomer? CH3 Ph
-
Draw a stereo isomer of this compound that is chiral, and draw two that are not chiral? CH3 -
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


