Propose a mechanism for the reaction of 1-chioroanthraquinone with methoxide ion to give the substitution product 1-methoxyanthraquinone.
Question:
Propose a mechanism for the reaction of 1-chioroanthraquinone with methoxide ion to give the substitution product 1-methoxyanthraquinone. Use curved arrows to show the electron flow in eachstep.
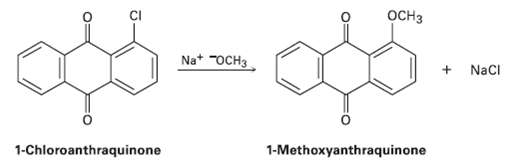
Transcribed Image Text:
OCH3 Na* "OCH3 Naci 1-Chloroanthraquinone 1-Methoxyanthraquinone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
0 CI OCH3 co Addition of the nucleophile OCH3 The carb...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
-
Propose a mechanism for the reaction of phenyl isocyanate with ethanol.
-
(a) Propose a mechanism for the reaction of benzyl alcohol with acetyl chloride to give benzyl acetate. (b) Propose a mechanism for the reaction of benzoic acid with acetyl chloride to give acetic...
-
What is the result of the following? A. 0 B. 0.007 C. The code does not compile due to line 7. D. The code does not compile due to line 8. E. The code does not compile for another reason. 1: import...
-
During 2016, Inez had the following transactions involving capital assets: Gain on the sale of unimproved land (held as an investment for 3 years)......................$ 3,000 Loss on the sale of a...
-
On a cool (4.0 o C) Saturday morning, a pilot fills the fuel tanks of her Pitts S-2C (a two-seat aerobatic airplane) to their full capacity of 106.0 L. Before flying on Sunday morning, when the...
-
Under what circumstances does a company prepare consolidated financial statements? AppendixLO1
-
The Fanta Company presents you with the following account balances taken from its December 31, 2007 adjusted trial balance: Inventory, January 1, 2007 ............. $ 43,000 Selling expenses...
-
Multiple Choice The shipping department is a service department for Ajax company that manufactures 2 products: scouring powder sold in shaker cans and a liquid detergent sold in 5-pound pails. The...
-
The Baldwin Company wants to decrease its plant utilization for Buzz by 15%. How many units would need to be produced next year to meet this production goal? Ignore impact of accounts payable on...
-
Draw resonance structures of the intermediate carbocations in the bromination of naphthalene, and account for the fact that naphthalene undergoes electrophilic substitution at C1 rather than C2. Br...
-
4-Chloropyridine undergoes reaction with dim ethylamine to yield 4-dimethylaminopyridine. Propose a mechanism for thereaction. CI N(CH3)2 HN(CH3)2 HCI N.
-
The following income statement items appeared on the adjusted trial balance of Schembri Manufacturing Corporation for the year ended December 31, 2024 ($ in thousands): sales revenue, $15,300; cost...
-
When in 1920 the Chia brothers opened their first shop in Bangkok selling seeds for farmers, they did not know that they were on the way to launching the development of one of the most successful...
-
In Exercises 49-52, sketch a plane. Then sketch the described situation. Three noncollinear points that lie in the plane
-
In Exercises 49-52, sketch a plane. Then sketch the described situation. A plane perpendicular to the given plane
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 150 F P G
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 30 B C
-
What did this case teach you about leadership?
-
Describe the general ways that the revised Form 990, applicable for tax year 2008 and beyond, is different from previous versions.
-
A Rankine power generation cycle is operated with water as the working fluid. It is found that 100 MW of power is produced in the turbine by 89 kg/s of steam that enters the turbine at 700 C and 5...
-
Explain which of the three products shown in is formed when 1-butene reacts with HCI.
-
Explain which of the four products shown in is formed when cis-2-pentene reacts with CI2 andwater.
-
Explain which of the four products shown in is formed when cyclopentene reacts with CI2 andwater.
-
A first-time shareholder has approached you requesting some advice. The shareholder has received the company's annual report and noticed the following statement in the summary of significant...
-
View Policies Current Attempt in Progress REI sells snowboards. Assume the following information relates to REI's purchases of snowboards during September. During the same month, 1 0 2 snowboards...
-
*Please explain how you got the answers* The following costs result from the production and sale of 1,000 drum sets manufactured by Tight Drums Company for the year ended December 31, 2019. The drum...

Study smarter with the SolutionInn App


