Propose a mechanism for the followingreaction: HO- CO2CH3 (CH3CH2)3N Heat BRCH2 CO2CH3
Question:
Propose a mechanism for the followingreaction:
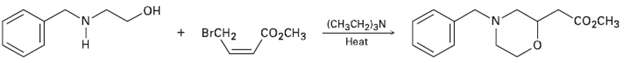
Transcribed Image Text:
HO- CO2CH3 (CH3CH2)3N Heat BRCH2 CO2CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
NH HC proton transfer N Br COCH...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for each reaction. (a) (b) (c) (d) OH H2SO heat OCH + CH3OH H20 CH2OH H2So4 heat CH OCH2CH CH CH,CH2OH (a minor product)
-
Propose a mechanism for the acid-catalyzed condensation of n-propyl alcohol to n-propyl ether, as shown above. When the temperature is allowed to rise too high, propene is formed. Propose a mechanism...
-
Propose a mechanism for the base-promoted hydrolysis of g-butyrolactone:
-
Suppose a single 802.11n client is connected to an 802.11n access point and there is no other client or access point in the neighborhood. The client senses the medium, then sends a 2000-Byte frame,...
-
What information or questions can you use when writing a follow-up message after submitting a résumé?
-
The March 31, 2020, adjusted trial balance for Amusement Park Repair is shown below with accounts in alphabetical order. Required Preparation Component: a. Place an X in the space provided beside...
-
McDonalds operates restaurants around the globe. To see how products vary, visit some of McDonalds international websites (www.mcdonalds.ie, www.mcdonalds.it, and www.mcdonalds.com.mx). Which product...
-
Fifty percent of the customers who go to Sears Auto Center for tires buy four tires and 30% buy two tires. Moreover, 18% buy fewer than two tires, with 5% buying none. a. What is the probability that...
-
Comprehensive Question - Becky's Car Detailing Services Becky Jones opened Becky's Car Detailing Services on April 1 , 2 0 2 7 . In Apr, the following transactions were completed. Apr - 0 1 Invested...
-
Read Case 14-1 Trojan Technologies (15th ed., p. 426 OR 16th ed., p. 431) Guiding Questions and additional information: In preparing your case study, ensure that you answer the following questions:...
-
The following transformation involves a conjugate nucleophilic addition reaction (Section 19.13) followed by an intra molecular nucleophilic acyl substitution reaction (Section 21.2). Show...
-
One step in the biosynthesis of morphine is the reaction of dopamine with p-hydroxyphenylacetaldehyde to give S)-norcoclaurine. Assuming that the reaction is acid-catalyzed, propose amechanism. . NH ...
-
E Chivers commenced business on 1 January 20X7 and makes his accounts to 31 December every year. For the year ended 31 December 20X7, bad debts written off amounted to 1,200. It was also found...
-
PP Company purchases a material that is then processed to yield three chemicals: anarol, estyl, and betryl.In June, PPC purchased 10,000 gallons of the material at a cost of $250,000, and the company...
-
Suppose Boyson Inc. free cash flow for the next year is $ 1 5 0 , 0 0 0 and the FCF is expected to grow a concert rate of 6 . 5 % if WACC is 1 2 . 5 % what is the market value of the firm?
-
An eight lane urban freeway (four lanes in each direction) is on rolling terrain and has 11-ft lanes with a 4-ft right-side shoulder. The interchange density is 1.25 per mile. The base free-flow...
-
For the following business events, please indicate the increase (+) or decrease (-) on the following income statement and balance sheet categories. If there is no effect, leave the box blank. If...
-
4. Change the magnet to the original orientation and drag through the coil. a. What happens to the voltage and light bulb as the North Pole moves through the coil? b. What happens to the voltage and...
-
Tawana purchased real property in 2008 at a cost of $200,000. In 2010, she is experiencing cash-flow problems and sells the property for $220,000. The adjusted basis of the property is $185,000.
-
Nike manufactures shoes and sportswear. How has the Internet changed the way this company communicates with its suppliers and retail customers?
-
Find the pH of a 0.0100 M sulfuric acid (H 2 SO 4 ) solution.
-
Write out the steps in the mechanism for the sulfonation of benzene.
-
Which product would you expect if propene were used in place of ethene in eq. 4.11 (or eqs. 4.20 and 4.21): propylbenzene or isopropylbenzene? Explain. Eq. 4.11 CH2CH3 H2SO alkylation (4.11)
-
Draw the important resonance contributors for the benzenonium intermediate in the bromination of aniline, and explain why ortho, para substitution predominates. NH2 aniline
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


