Question: A published study of a chemical reaction A ? P, indicates that if the reactor initially contains A at a concentration C A0 (g/L) and
A published study of a chemical reaction A ? P, indicates that if the reactor initially contains A at a concentration CA0(g/L) and the reaction temperature, T, is kept constant, then the concentration of P in the reactor increases with time according to the formula Cp(g/L) = CA0 (1 ? e?kt). The rate constant, k (s ? 1), is reportedly a function only of the reaction temperature. To test this finding, the reaction is run in four different laboratories. The reported experimental results are given below:
(a) What plot would yield a straight line if the given equation is correct?
(b) Enter the given data into a spreadsheet. For each data set (CP versus t), generate the plot of part (a) and determine the corresponding value of k. (Your spreadsheet program probably has a built-in function to perform a linear regression on the data in two specified columns).
(c) Use the results in part (b) to come up with a good estimate of the value of k at 275oC. Explain how you did it.
(d) If you did the calculation in part (b) correctly, one of the calculated values of k should be considerably out of line with the others. Think of as many possible explanations for this result as you can (up to 10)
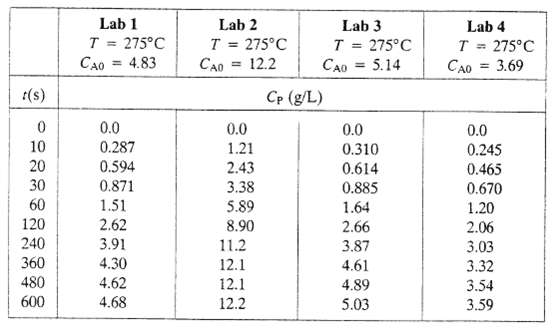
t(s) 0 10 20 30 60 120 240 360 480 600 Lab 1 T = 275C 4.83 CAO 0.0 0.287 0.594 0.871 1.51 2.62 3.91 4.30 4.62 4.68 Lab 2 T = 275C 12.2 CAO = 0.0 1.21 2.43 3.38 5.89 8.90 11.2 12.1 12.1 12.2 Lab 3 T 275C 5.14 CAO Cp (g/L) = 0.0 0.310 0.614 0.885 1.64 2.66 3.87 4.61 4.89 5.03 Lab 4 T = 275C = 3.69 CAO 0.0 0.245 0.465 0.670 1.20 2.06 3.03 3.32 3.54 3.59
Step by Step Solution
3.52 Rating (172 Votes )
There are 3 Steps involved in it
a In1CpC40 vs t in rectangular coordinates Slopek intercept0 b In1CpCao ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (42).pdf
180 KBs PDF File
13-E-C-E-C-P (42).docx
120 KBs Word File


