The heat capacities of a substance have been defined as use the defining relationship between H and
Question:
The heat capacities of a substance have been defined as use the defining relationship between H and U and the fact that H and U for ideal gases are functions only of temperature to prove that Cp = Cv + R for an idealgas.
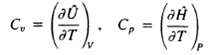
Transcribed Image Text:
C. a0 aT CP = ай ат
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 38% (13 reviews)
PVRT PV RT But sinc...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:
Students also viewed these Chemical Engineering questions
-
Would Alberty have been defined as an employee if she had been a foreign correspondent who reported on stories from international locations and WIPR had paid her travel and related expenses? Explain.
-
In describing the heat capacities of solids in Section 18.4, we stated that the potential energy U = 1/2kx2 of a harmonic oscillator averaged over one period of the motion is equal to the kinetic...
-
An ideal gas, CP = {5f2) R and CV = (3/2) R, is changed from P = 1 bar and v11 = 12 m3 to P2 = 12 bar and v12 = 1 m3 by the following mechanically reversible processes: (a) Isothermal compression (b)...
-
If the Federal Government increases taxes:What will be the effect on money demand, money supply, and interest rates? Money demand decreases, money supply is unchanged, and interest rates decrease...
-
Explain why the valuation by components approach can save computational time and still lead to the correct answer.
-
The paper "Exercise Thermoregulation and Hyperprolac-tinaemia" (Ergonomics, 2005: 1547-1557) discussed how various aspects of exercise capacity might depend on the temperature of the environment. The...
-
IQ and reading scores Data on the IQ test scores and reading test scores for a group of fifth-grade children give the following regression line: predicted reading score = 33.4 + 0.882(IQ score). (a)...
-
Income statement information for Einsworth Corporation is provided below. Sales ..........$1,200,000 Cost of goods sold..... 780,000 Gross profit........ 420,000 Prepare a vertical analysis of the...
-
Aug. 1 Madison Harris, the owner, invested $7,500 cash and $34,500 of photography equipment in the company in exchange for common stock. 2 The company paid $3,100 cash for an insurance policy...
-
A. Russell (birthdate February 2, 1967) and Linda (birthdate August 30, 1972) Long have brought you the following information regarding their income and expenses for the current year. Russell owns...
-
The heat required to raise the temperature of m (kg) of a liquid from T 1 to T 2 at constant pressure is in high school and in first-year college physics courses, the formula is usually given as (a)...
-
Ralph Rack straw your next-door neighbor, surprised his wife last January by having a hot tub installed in their back yard while she was away on a business trip. It surprised her, all right, but...
-
At 35oC, K = 1.6 10-5 for the reaction 2NOCl(g) 2NO(g) + Cl2(g) Calculate the concentrations of all species at equilibrium for each of the following original mixtures. a. 2.0 moles of pure NOCl in...
-
On Apple company with specific iPhone product Required to conduct a SWOT and PESTEL analysis, identifying the internal strengths and weaknesses and external opportunities and threats of the Apple...
-
In which social platforms are Walmart's brand/company active? In your opinion, are they doing a good job regarding customer engagement through social media channels? (Required: screenshots from the...
-
After you have watched both films, how would you describe each film? Also, consider what makes these early films different. List as many observations as you can that separate the Lumi re brothers...
-
How to develop the following points with the Poshmark application for second hand? 1. What are the main reasons for using this product? Or why not? 2. What are the hidden motivations? 3. Are there...
-
Suppose, in an experiment to determine the amount of sodium hypochlorite in bleach, you titrated a 22.84 mL sample of 0.0100 M K I O 3 with a solution of N a 2 S 2 O 3 of unknown concentration. The...
-
Identify the two fundamental functions involved in the operation of the insurance mechanism.
-
Identify the Critical Infrastructure Physical Protection System Plan.
-
Find a parametric representation of the sphere x 2 + y 2 + z 2 = a 2
-
Cold air at 20F, 760 mm Hg pressure and 70% relative humidity is conditioned by being passed through a bank of heatin2 coils, then through a water spray and finally through a second set of heating...
-
Spray cooling is a technique for cooling and either humidifying or dehumidifying air by contacting it with a liquid water spray. The liquid water leaving the tower is re-circulated and, in the case...
-
The heat of solution of ammonia in water at 1 atm is Hs (25C, r = 2 mol H2O/mol NH3) = 78.2 kJ/mol Calculate the enthalpy change that accompanies the dissolution of 400 mol of NH3 in 800 mol of water...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...
-
Horizontal Analysis The comparative accounts payable and long-term debt balances of a company are provided below. Current Year Previous Year Accounts payable $47,286 $63,900 Long-term debt 85,492...
-
On January 1, Year 1, Price Company issued $140,000 of five-year, 7 percent bonds at 97. Interest is payable annually on December 31. The discount is amortized using the straight-line method. Record...

Study smarter with the SolutionInn App


