Question: Relatively pure oxygen and nitrogen can be obtained by the distillation of air using the Linde double column, which, as shown in Figure, consists of
Relatively pure oxygen and nitrogen can be obtained by the distillation of air using the Linde double column, which, as shown in Figure, consists of a lower column operating at elevated pressure surmounted by an atmospheric-pressure column. The boiler of the upper column is at the same time the reflux condenser for both columns. Gaseous air plus enough liquid to take care of heat leak into the column (more liquid, of course, if liquid-oxygenproduct is withdrawn) enters the exchanger at the base of the lower column and condenses, giving up heat to the boiling liquid and thus supplying the vapor flow for this column. The liquid air enters an intermediate point in this column, as shown in Figure. The vapors rising in this column are partially condensed to form the reflux, and the uncondensed vapor passes to an outer row of tubes and is totally condensed, the liquid nitrogen collecting in an annulus,as shown. By operating this column at 4 to 5 atm, the liquid oxygen boiling at 1 atm is cold enough to condense pure nitrogen. The liquid that collects in the bottom of the lower column contains about 45 mol% O2 and forms the feed for the upper column. Such a double column can produce very pure oxygen with high oxygen recovery, and relatively pure nitrogen. On a single McCabe-Thiele diagram-using equilibrium lines, operating lines, q-lines, 45oline, stepped-off stages, and other illustrative aids-show qualitatively how the stage requirements of the double column can becomputed.
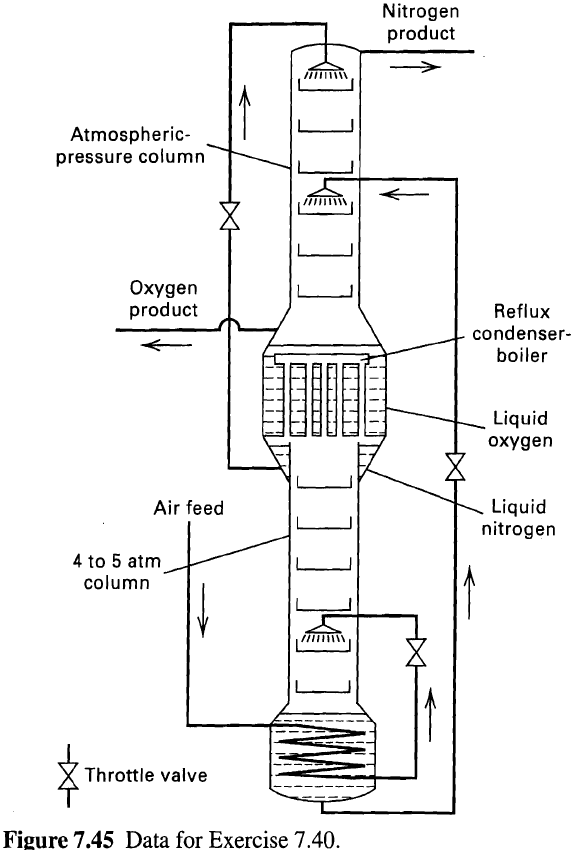
Nitrogen product Atmospheric- pressure column Oxygen product Reflux condenser- boiler Liquid en Liquid nitrogen Air feed 4 to 5 atm column Throttle valve Figure 7.45 Data for Exercise 7.40.
Step by Step Solution
3.48 Rating (181 Votes )
There are 3 Steps involved in it
In LC the N 2 composition ranges from 55 mol at the bottom to about 99 mol at the top with a ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (284).docx
120 KBs Word File


