Question: Repeat Example 5.4 for the following numbers of equilibrium stages (see Figure): (a) M = 10, N = 10 (b) M = 15, N =
Repeat Example 5.4 for the following numbers of equilibrium stages (see Figure):
(a) M = 10, N = 10
(b) M = 15, N = 15
Plot dc3/bc3 as a function of M + N from 10 to 30 stages. In making the calculations, assume that state temperatures and total flow rates do not change from the results of Example 5.4. Discuss the effect of the number of stages on theseparation.
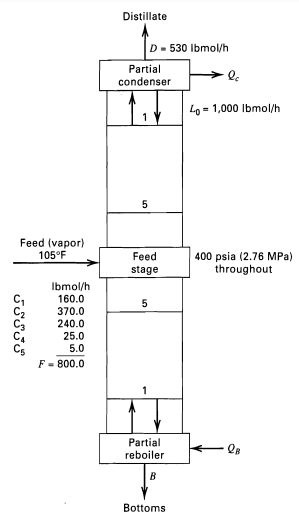
Distillate D- 530 Ibmol/h Partial condenser Lo = 1,000 Ibmol/h 5 Feed (vapor) 105F Feed 400 psia (2.76 MPa) throughout stage Ibmol/h 160.0 370.0 240.0 25.0 5.0 F = 800.0 Partial reboiler QB Bottoms
Step by Step Solution
3.26 Rating (167 Votes )
There are 3 Steps involved in it
Using the Edmister group method the ratio of d to b for any component is g... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (186).docx
120 KBs Word File


