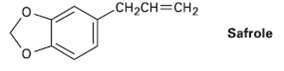
Safrole, a substance isolated from oil of sassafras, is used as a perfumery agent. Propose a synthesis
Question:
Safrole, a substance isolated from oil of sassafras, is used as a perfumery agent. Propose a synthesis of safrole from catechol (1,2-benzenediol).
Transcribed Image Text:
CH2CH=CH2 Safrole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
OH OH 2 NaH O ...View the full answer

Answered By

Shyamsunder K
Teaching my kids and students ( though I am not a teacher by profession) around me and helping them in easily comprehend the subjects; though teaching is my passion but not as profession and students who I have taught have achieved success in taking the exams confidently and perform great;y.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Karahanaenone, a terpenoid isolated from oil of hops, has been synthesized by the thermal reaction shown. Identify the kind of pericyclic reaction, and explain how karahanaenone isformed. CH CH eat...
-
Safrole is contained in oil of sassafras and was once used to flavor root beer. A 2.39-mg sample of safrole was dissolved in 103.0 mg of diphenyl ether. The solution had a melting point of 25.70C....
-
Propose a formula for hydrogen peroxide, a substance used as a bleaching agent. (Curiously, this compound does not behave as an acid, despite its formula. It behaves more like a classic...
-
The following financial statements for Brownstone plc are a slightly simplified set of published accounts. Brownstone plc is an engineering business that developed a new range of products in 2007....
-
Evaluate how Apple can gain business intelligence through the implementation of a customer relationship management system.
-
What information related to the companys property, plant, and equipment must be presented in the financial statements and in the notes to the financial statements?
-
2. In the preparation of consolidated financial statements, intercompany items for which eliminations will not be made are: a Purchases and sales where the parent employs the equity method b...
-
How are the six steps in the data-driven approach to fraud detection different from the traditional reactive approach?
-
Please solve 1 - 6 * Applied to Work in Process during the period. The company's manufacturing overhead cost is applied to production on the basis of direct labor - hours. All of the materials...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Meerwein?s reagent, triethyloxonium tetra-fluoroborate, is a powerful ethylating agent that converts alcohols into ethyl ethers at neutral pH. Show the reaction of Meerwein?s reagent with...
-
Epoxides are reduced by treatment with lithium aluminum hydride to yield alcohols. Propose a mechanism for thisreaction. 1. LIAIH4, ether 2. H*
-
Briefly describe the Working Capital Guarantee Program administered by the Export-Import Bank.
-
For the past 30 years, the average satisfaction rating for a sushi restaurant has been 3.9 out of 5. If the rating for a sample of 256 people is 4.1 with a standard deviation of 0.5, the critical...
-
Hash collisions occur when more than one item is mapped to the same element in Hash Table's array. What is one way that a Hash Table can handle collisions?
-
Scatterplot. In Exercises 5-8, use the sample data to construct a scatterplot. Use the first variable for the x-axis. Based on the scatterplot, what do you conclude about a linear correlation? Pulse...
-
Given two fair six sided dice and a standard deck of 52 playing cards, calculate the probability of a rolling a sum of 7 or 11 and drawing three cards in which at least one is a face card.
-
z Scores. In Exercises 5-8, express all z scores with two decimal places. 5. Diastolic Blood Pressure of Females For the diastolic blood pressure measurements of females listed in Data Set 1 "Body...
-
Use the Internet to look up and identify whether tip pooling is permitted in the state where you work or go to school. Cite your information source and the path to get there. AppendixLO1
-
Refer to the data in QS 10-1. Based on financial considerations alone, should Helix accept this order at the special price? Explain.
-
A tank contains 20 percent liquid water and 80 percent steam by volume at 200 C. Steam is withdrawn from the top of the tank until the fluid remaining in the tank is at a temperature of 150 C....
-
Consider another chemical species like the ones in the previous problems in which a carbon atom forms three single bonds to three hydrogen atoms but in which the carbon atom possesses a single...
-
Draw a three-dimensional orbital representation for each of the following molecules, indicate whether each bond in it is a s or p bond, and provide the hybridization for each non-hydrogen atom. (a)...
-
Ozone (O3) is found in the upper atmosphere where it absorbs highly energetic ultraviolet (UV) radiation and thereby provides the surface of Earth with a protective screen. One possible resonance...
-
Production costs that are not attached to units that are sold are reported as: Cost of goods sold Selling expenses Administrative costs Inventory
-
Please show workings :) Oxford Company has limited funds available for investment and must ration the funds among four competing projects. Selected information on the four projects follows: Life of...
-
ASDA Company reported the following activity in the Assembly Department for the month of May : Units Percent Completed Materials Conversion Work in process, May 1 360 50 % 10 % Units started into...

Study smarter with the SolutionInn App


