Show the product of the Diels?Alder reaction of the following diene with 3-buten-2-one, H 2 C =
Question:
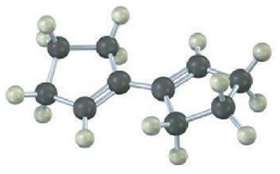
Show the product of the Diels?Alder reaction of the following diene with 3-buten-2-one, H2C = CHCOCH3. Make sure you show the full stereochemistry of the reaction product.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
strans ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the product of thisreaction: Ph Br- - + NaOEt Br EIOH Ph
-
Show how you would make each compound, beginning with an alcohol of your choice. (a) (b) (c) (d) (e) (f) (g) (h) CHO CH,Br CI CH CH 1 CH C OH CH3 OTs
-
Show how you might use a nucleophilic substitution reaction of 1-bromopropane to synthesize each of the following compounds. (You may use any other compounds that are necessary.) (a) (b)...
-
What is the discount yield, bond equivalent yield, and effective annual return on a $ 5 million commercial paper issue that currently sells at 98.625 percent of its face value and is 136 days from...
-
Suppose a small translation business asked you to advise a company on how to overcome cultural barriers among a staff drawn from three countries. Suggest a few ways the company could use training and...
-
United Steel manufactures two types of steel at three different steel mills. During a given month, each steel mill has 200 hours of blast furnace time available. Because of differences in the...
-
Differentiate the decision conditions of certainty, risk, and uncertainty.
-
Your company, Acme Widgets, sells its widgets worldwide. Acme has a contract for 250,000 widgets to be shipped to the Czech Republic. The price stated in the offer and acceptance is $1 per widget,...
-
TMB 3. In recent times bank is witnessing volatility in interest rates as well as foreign exchange rates. Thus putting pressure on the banks for maintaining a good balance among spreads,...
-
Powersys is an electricity distribution company based in a large capital city. Its business is to manage the electricity assets, including poles, wires, and other equipment, that are used to deliver...
-
Show the structures of all possible adducts of the following diene with 1 equivalent ofHC1:
-
The following diene does not undergo Diels?Alder reactions. Explain.
-
Refer to your solutions for Sigrids Custom Graphics in PB5-2. Required: 1. Consider the pattern of the companys activity and costs throughout the year. Would you consider this to be a seasonal...
-
Construct a 90% confidence interval for the population standard deviation o at Bank B. Bank B 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Jamila Traders has a head office in Nanyuki and an autonomous branch in Thika. The trial balances of the head office and the branch as at 30 September 2014 were as follows: Head office Sh. Sh. Thika...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
ROI analysis using the DuPont model a. Firm A has a margin of 7%, sales of $980,000, and ROI of 19.6%. Calculate the firm's average total assets. b. Firm B has net income of $259,200, turnover of...
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
Go to http://www.shodor.org/interactivate/activities/piechart/. Prepare a report explaining how the information helps you to better understand pie charts.
-
Consider the circuit of Fig. 7.97. Find v0 (t) if i(0) = 2 A and v(t) = 0. 1 3 ett)
-
How does the solubility of a gas in a liquid depend on pressure? How does this pressure dependence account for the bubbling that occurs upon opening a can of soda?
-
What products would you expect from the following reactions? (a) (b) PdC PPh3. Et N Br Br Pd(OAc)h PPhy. EtyN
-
What substituted alkene would you use in the Heck reaction to make the following products? (a) (b) OCH3 Pd(OAc)2 PPh3, EtyN CH CH3 CN Br Pd(OAch PPh. ElN
-
p-Xylene undergoes nitration much faster than benzene. Use resonance forms of the sigma complex to explain this accelerated rate.
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases

Study smarter with the SolutionInn App


