Show the products of thesereactions: H,SO,, H,SO4 b) + H,0 + H,0 H,SO. + HO
Question:
Show the products of thesereactions:
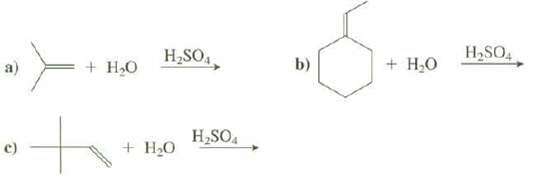
Transcribed Image Text:
H,SO,, H,SO4 b) + H,0 + H,0 H,SO. + HO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
These are acid catalyzed hydrolysis reactions where th...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the products of these reactions. (Remember that acid-base reactions are usually much faster than nucleophilic substitution reactions.) a) C1-CHCHCHCOH b) Br OH NH3 + OH
-
Show the products of these reactions and explain whether each would follow an SN1 or an SN2 mechanism: a) C) B CI + OH Br + SH DMF CHOH HO Br + HO CHOH HO b) d) f) CI + HO CH,OH OTS + CH0 Br + CHCO...
-
Show the products of these eliminationreactions: CH3 ELOH + NaOCH,CH3 a) "CI Br ELOH + NaOCH,CH; b) "CH,CH
-
Assume a retailer has fixed costs of $10,000, a unitvariable cost of $25, and a 50% retail margin. How many units must be sold for her tobreak-even? If she has a target profit of $200,000, how many...
-
Faced with rising pressure for a $15 per hour minimum wage rate, the farming industry is currently exploring the possible use of robotics to replace some farm workers. The Lettuce Bot is one such...
-
American Apparel has been in the news in recent months. Its board fired CEO Dov Charney amid several reports of his misdeeds. The company has also lost $270 million over the past three years. Last...
-
Individuals who report perceived wrongdoing of a corporation or public agency are known as whistle blowers. Two researchers developed an index to measure the extent of retaliation against a whistle...
-
From the following information, draw the project network. Compute the early, late, and slack times for each activity. Identify the critical path. (Draw the finish-to-start relationshipsfirst.)...
-
P 5 - 6 ( Algo ) Determining Bad Debt Expense Based on Aging Analysis and Interpreting Ratios LO 5 - 3 , 5 - 4 IceKreme Inc. makes ice cream machines for sale to ice cream parlours. The following...
-
Draw the shear and moment diagrams for each member of the frame. Assume the frame is pin connected at B, C, and D and A is fixed. 6 k 6 k 3 k 3 k 8 ft - 8 ft 8 ft 0.8 k/ft 15 ft
-
Show the products of thesereactions: CH3 HBr a) CH,CHCH-CH2 HBr b) PhC CH 2 HBr
-
Show all of these steps in the mechanism for the addition of water to propene catalyzed by sulfuric acid. Explain whether propene or phenylethene (PhCH = CH2) has a faster rate in this reaction:
-
Why might an organization prefer to use positive reinforcement rather than negative reinforcement?
-
From your reading this unit on motivation and change from the TIP series, what is the connection and interplay between these concepts/statements below in your opinion in working with clients facing...
-
Please help with the following The partnership of Bauer, Ohtani, and Souza has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 276,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Define meaning of partnership deed.
-
List down the information contains in the partnership deed.
-
Define craft and industrial unions, and outline the sequence of events in organizing and certifying or decertifying a union. (pp. 336-342)
-
7 A 29-year-old, previously healthy man suddenly collapses at a party where legal and illicit drugs are being used. Enroute to the hospital, he requires resuscitation with defibrillation to establish...
-
Calculate the molar solubility of Co(OH) 3 , K sp = 2.5 10 -43 .
-
How would you prepare the following compounds from benzene, using a diazonium replacement reaction in your scheme? (a) p-Bromobenzoic acid (b) m-Bromobenzoic acid (c) n-Bromo chloro benzene (d)...
-
Propose a synthesis of p-(dim-ethylamine) azobenzene from benzene as your only organic starting material.
-
Draw an orbital picture of thiazole. Assume that both the nitrogen and sulfur atoms are sp2-hyhridized, and show the orbitals that the lone pairs occupy.
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
Which of the following statement regarding a post-closing trial balance is not true
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions

Study smarter with the SolutionInn App


