Show the rearranged carbocations that are expected from these carbocations: a) +CH CH3 b) CHCHCHCHCH c) CH3
Question:
Show the rearranged carbocations that are expected from these carbocations:
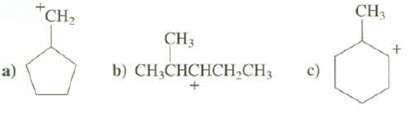
Transcribed Image Text:
a) +CH₂ CH3 b) CH₂CHCHCH₂CH₂ c) CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
A carbocation may rearrange to form a more stable ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show that a conditionally convergent series can be rearranged so to diverge?
-
Carbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secndary. Primary and methyl cations are too unstable to form in solution.
-
The acid-catalyzed dehydration of an alcohol to a rearranged alkene is known as a Wagner-Meerwein rearrangement. Propose a mechanism for the following Wagner-Meerwein rearrangement: OH ...wr + H30+...
-
1. What is Ladures target market and retail strategy in the United States? 2. Explain the reasons Ladure owns its stores in some countries and uses franchising with local licensees in others. 3....
-
Orbital Industries Ltd manufactures a variety of materials and equipment for the aerospace industry. A team of P & D engineers in the firm's Technology Park plant has developed a new material that...
-
Rita Pelzer was just hired as the assistant controller of Liu Stores. Th e company is a specialty chain store with nine retail stores concentrated in one metropolitan area. Among other things, the...
-
What are some of the principal reasons why project management has become such a popular business tool in recent years?
-
The factor of safety for tipping of the concrete dam is defined as the ratio of the stabilizing moment about O due to the dams weight divided by the overturning moment about O due to the water...
-
Copyright protects: ideas not the expression of the ideas the form of expression not the underlying idea ideas and inventions secrets
-
A Global private bank is aggressively looking to leverage technology to improve customer experience and reduce operational costs. Over the last few years, it has tied up with at least five startups...
-
Show both the substitution and elimination products that are formed in these reactions: a) C CI + CH0 CHOH + OH HO EtOH b) Br + CHOH CH3OH
-
Show the substitution products for these reactions: a) b) +CHCHOH Br CI Ja + HO EtOH HO EtOH
-
(Prepare a cash flow statement, LO 5) Markham Ltd. was organized on September 1, 2004 with a cash investment of $700,000 by its shareholders. Markham arranged a long-term loan with a local bank for...
-
Using a ruler and set squares only, construct the following shapes: a. b. c. d. 5cm 5cm
-
The marketing department has just forecast that 10,000 units of item 778 will be ordered in the next fiscal year. Based on the marketing department's forecast and noting that the seasonal relative...
-
Following are interaction plots for three data sets. Which data set has the largest interactions? Which has the smallest? A B C
-
From your local chamber of commerce, obtain the population figures for your city for the years \(1980,1990,2000\), and 2010. Find the rate of growth for each period. Forecast the population of your...
-
A mass \(m\) is attached at the midpoint of a stretched wire of area of cross-section \(A\), length \(l\), and Young's modulus \(E\) as shown in Fig. 13.29. If the initial tension in the wire is...
-
Lemon Inc. is a company registered in the U.K. which produces computers and has a call center registered in the Republic of H to address complaints associated with its computer products. The standard...
-
(a) As Section 17.3 discusses, high-frequency sound waves exhibit less diffraction than low-frequency sound waves do. However, even high-frequency sound waves exhibit much more diffraction under...
-
Solve each inequality and graph its solution. -3b + 5 + 7 < 14-b -9-8-7-654-3 -2 -1 0 1
-
Draw and name all monochloro products you would expect to obtain from radical chlorination of 2-methylpentane. Which, if any, are chiral?
-
Taking the relative reactivities of 1 o , 2 o , and 3 o hydrogen atoms into account, what product(s) would you expect to obtain from monochlorination of 2-methylbutane? What would the approximate...
-
Draw three resonance forms for the cyclohexadienylradical. Cyclohexadienyl radical
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


