In the figure, AB CD and BC DE. What is the value of x? A)
Question:
In the figure, A̅B̅ ∥ C̅D̅ and B̅C̅ ∥ D̅E̅. What is the value of x?
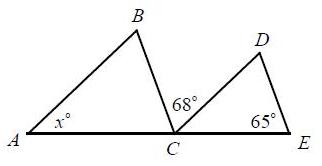
A) 47
B) 51
C) 55
D) 57
Transcribed Image Text:
A B 68° C D 65° E
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
A A B mBCAmDEC mBCA 65 mDCE x 68 C Note Figure no...View the full answer

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
FIGURE EX9.8 is the kinetic-energy graph for a 2.0 kg object moving along the x-axis. Determine the work done on the object during each of the four intervals AB, BC, CD, and DE. K (J) 4- 2 A B C D E...
-
1. How many moles of magnesium is 3.01 x 1022 atoms of magnesium? 2. Find the mass in 2.6 mol of lithium bromide. 3. Use the equation below to answer questions 3a and 3b. C6H12O6 ->2CH5OH + 2CO2 a....
-
if we choose statistic as our keyword, our cipher would be determined as follows: method i. write the word statistic without the repeated letters. then complete the cipher with the unused alphabet...
-
Pierce Phones is considering the introduction of a new model of headphone whose selling price is $18 per unit and whose variable expense is $15 per unit. The company's monthly fixed expense is...
-
Financial information related to Oak Tree Interiors for October and November 2012 is as follows: a. Prepare balance sheets for Oak Tree Interiors as of October 31 and as of November 30, 2012. b....
-
The Na + ion and the Ne atom are isoelectronic. The ease of loss of an electron by a gaseous Ne atom, first ionization energy, has a value of 2081 kJ/mol. The ease of loss of an electron from a...
-
Explain contractual entry strategies. LO.1
-
Cartwell Inc. makes picture frames which are sold in a local retail store and through various websites. Wood for frames .......................................................... $10,000 Rent for...
-
How do auditors assess the going concern assumption of a business?
-
The graph depicts a survey of 400 senior students in a high school who took the AP tests last May. The number of AP tests taken by each student ranges from zero to six. By what percent is the number...
-
Effective December 31, 2023, Zintel Corporation proposes to issue additional shares of its common stock in exchange for all the assets and liabilities of Smith Corporation and Platz Corporation,...
-
Based upon the information presented in the problem, conduct an analysis of the client's case and prepare a complete and detailed analysis of the problem.
-
Each of the accompanying graphs shows a do plot of data from three separate random samples for each of the four graphs, indicate whether you think that the basic assumptions for single-factor ANOVA...
-
Program Milestones Milestone #1 - Selection GUI - Create Account/Login/Cancel Obtain a copy of Eclipse and complete "Getting Started in Eclipse". Create your project in Eclipse and the package and...
-
= The momentum transfer is q = ph - Ph, where p is the hadron momentum after the collision. This relationship holds for the time as well as the space components, i.e., for the 4-vectors. Thus, we...
-
When should HR be the interviewer, and when should a hiring manager or co-workers be involved? Should reference checking be done before or after the interview? Would you ask during the interview any...
-
1. Monoclean Company manufactures a single product, Glamour. The standard cost specification sheet shows the following standards for one unit of Glamour: 8 kg of material M @ $6.5 per kg $52 4 hours...
-
There is no doubt that Microsoft Office (including Access & Excel) is the industry leader for office productivity software - it can be found in almost all businesses. But, recently there has been a...
-
What is the mode?
-
Working for ones self generally means that the amount of time devoted to work is up to the individual. Is there sufficient evidence to conclude that people who work for themselves (WRKSLF: 1 = Self ,...
-
There appear to be many attractive features of government jobs, the most attractive being job security. But is this actually the case? Conduct a test to determine whether government workers (WRKGOVT:...
-
Do men (SEX: 1 = Male, 2 = Female) prefer jobs with higher incomes more than do women? Conduct a statistical test to answer the question. Please look at the list below and specify which one you would...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


