A stoichiometric mixture of hydrogen and air is compressed to 18.63 bar and (845^{circ} mathrm{C}). It burns
Question:
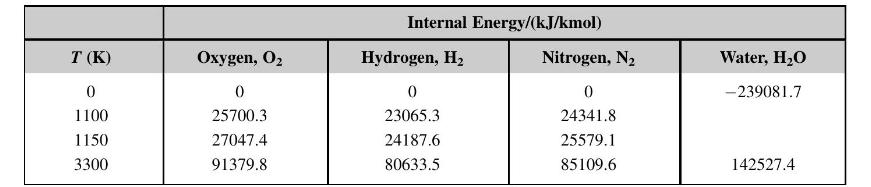
A stoichiometric mixture of hydrogen and air is compressed to 18.63 bar and \(845^{\circ} \mathrm{C}\). It burns adiabatically at constant volume. Show that the final equilibrium temperature is \(3300 \mathrm{~K}\) and the degree of dissociation is \(8.85 \%\). Calculate the final pressure after combustion. Show the process, including the effect of dissociation, on a \(U-T\) diagram. The equilibrium constant is
\[\begin{aligned}K_{p_{\mathrm{r}}} & =\frac{\left(p_{\mathrm{H}_{2} \mathrm{O}} / p_{0}\right)}{\left(p_{\mathrm{H}_{2}} / p_{0}\right)\left(p_{\mathrm{O}_{2}} / p_{0}\right)^{1 / 2}}=12.1389 \\\text { at } T & =3300 \mathrm{~K}\end{aligned}\]
Use the following internal energies
[47.58 bar]
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





