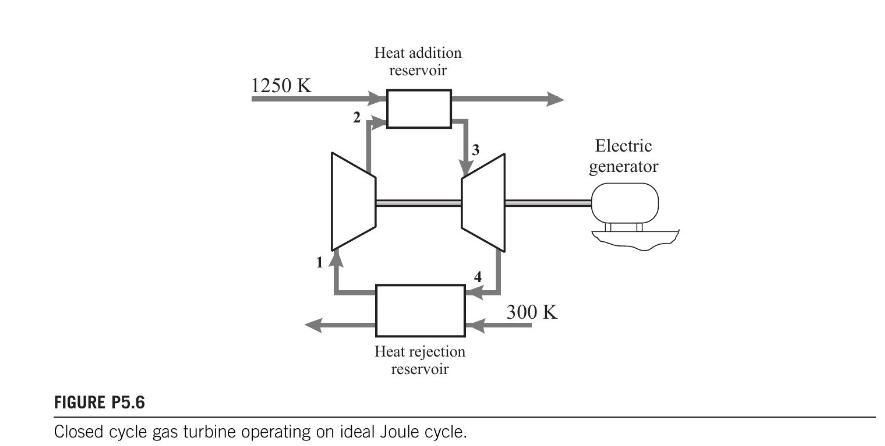
depicts a closed cycle gas turbine operating on the Joule cycle (i.e. constant pressure heat addition and
Question:
depicts a closed cycle gas turbine operating on the Joule cycle (i.e. constant pressure heat addition and rejection, and isentropic compression and expansion). Energy is added to the working fluid (air) by a heat exchanger maintained at \(1250 \mathrm{~K}\), and rejected to another heat exchanger maintained at \(300 \mathrm{~K}\). The maximum temperature of the working fluid is \(1150 \mathrm{~K}\) and its minimum temperature is \(400 \mathrm{~K}\). The pressure ratio of the compressor is \(5: 1\). Evaluate the irreversibilities introduced by the heat transfer processes and calculate
(a) the First Law efficiency \(\eta_{\mathrm{I}}=\frac{\text { work output }}{\text { energy addition }}\);
(b) the Second Law efficiency \(\eta_{\text {II }}=\frac{\text { work output }}{\text { availability of energy addition }}\).
Assume \(c_{p}=1.005 \mathrm{~kJ} / \mathrm{kg} \mathrm{K}, \kappa=1.4\), and the specific gas constant, \(R=0.287 \mathrm{~kJ} / \mathrm{kg} \mathrm{K}\).
Calculate the maximum efficiency that could be achieved from this system by modification of the heat exchangers.
[36.9\%; 48.5\%]
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





