Closed cycle gas turbines operate on the internally reversible Joule cycle with an efficiency of [eta_{text {Joule
Question:
Closed cycle gas turbines operate on the internally reversible Joule cycle with an efficiency of
\[\eta_{\text {Joule }}=1-\frac{1}{r_{p}^{(\kappa-1) / \kappa}}\]
where \(r_{p}=\) pressure ratio of the turbine and \(\kappa=\) ratio of specific heats, \(c_{p} / c_{v}=1.4\).
This equation significantly overestimates the efficiency of the cycle when external irreversibilities are taken into account. Shown in Fig. P6.3 is a \(T-s\) diagram for a closed cycle gas turbine receiving energy from a high-temperature reservoir at \(T_{\mathrm{H}}=1200 \mathrm{~K}\) and rejecting energy to a low-temperature reservoir at \(T_{\mathrm{C}}=400 \mathrm{~K}\).
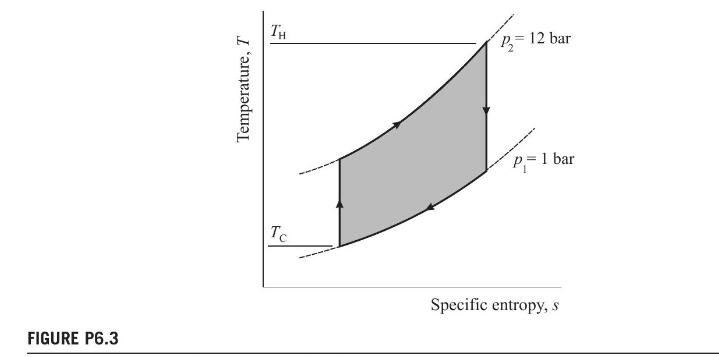
1. Evaluate the Carnot efficiency and compare this with the Joule efficiency; explain why the Joule efficiency is the lower.
2. Calculate the ratio of the gas turbine cycle work to the energy delivered from the hightemperature reservoir ( \(Q_{\mathrm{H}}\) for the Carnot cycle). This ratio is less than the Joule efficiency explain why in terms of unavailable energy.
3. Calculate the external irreversibilities and describe how these may be reduced to enable the Joule efficiency to be achieved.
4. What are the mean temperatures of energy addition and rejection in the Joule cycle? What would be the thermal efficiency of a Carnot cycle based on these mean temperatures?
\(\left[0.667 ; 0.508 ; 0.4212 ; I_{H} / c_{p}=79.9 ; 994 \mathrm{~K} ; 489 \mathrm{~K}\right]\)
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





