An absorber is designed to treat 40 kmol/h of air containing acetone with a mole fraction of
Question:
An absorber is designed to treat 40 kmol/h of air containing acetone with a mole fraction of 0.02 and reduce the mole fraction to 0.001. Pure water is the solvent, at a rate of 20 kmol/h. The column temperature is 26.7°C, and the pressure is 1 atm. Raoult’s law may be assumed to apply, and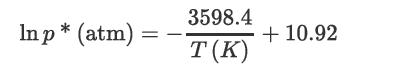
1. How many equilibrium stages are needed?
2. Assume that a column with five equilibrium stages is built and operational. It is now observed that the outlet mole fraction of acetone in the air is 0.002. Describe and explain at least four possible causes of this situation. Quantify what is happening in the column for each case.
3. Suggest realistic ways to compensate for this problem. An example of a nonrealistic compensation method is to reduce the air feed, which is probably fixed upstream.
4. The column is operating normally, but you are told to expect a 10%
increase in air to be treated. How can you change column operation to ensure the desired outlet mole fraction of acetone in air? Be quantitative.
5. The column is operating normally, but you are told to expect an inlet mole fraction of acetone of 0.025. How can you change column operation to ensure the desired outlet mole fraction of acetone in air? Be quantitative.
6. The column is operating normally, but you are told that the outlet mole fraction of acetone must be reduced to 0.00075. How can you change column operation to ensure the desired outlet mole fraction of acetone in air? Be quantitative.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





