Consider the removal of H 2 S only from air. The solvent has a molecular weight of
Question:
Consider the removal of H2S only from air. The solvent has a molecular weight of 300, μ = 2.5 × 10-3 kg/m/s, σ = 30 dyne/cm2, and SG = 0.90. The partition coefficient for H2S between air and solvent is given by the relationship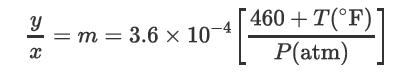
The problem at hand is the removal of H2S from an air stream initially containing 0.10% H2S, so that the exit gas only contains 0.0050% H2S. The absorber operates at an average pressure of 6 atm and an average temperature of 50°F. The inlet H2S content of the solvent can be assumed to be zero. The gas to be treated is at 200 lbmol/h, and it may be assumed that the solvent circulates at 2100 lb/h.
1. How many equilibrium stages are needed for this separation?
2. There is a problem with the solvent pump, which must be taken off-line soon, and the spare pump can only provide solvent at 5 atm at a flowrate no higher than 105% of the original flowrate. Therefore, the feed gas is to be throttled to 5 atm. Since the heat exchangers for this process already uses refrigerated water, 50°F is the lowest possible temperature. You ask two summer interns to evaluate the situation. One says that everything should be okay. The other says that the absorber will not work. Which intern would you hire?
3. What is the diameter of a sieve-tray column for this absorber with 24-in tray spacing for 70% of flooding with an active area of 90% of the total area?
4. If the trays are 20% efficient, is the height/diameter ratio within typical limits? Explain.
5. Estimate the pressure drop in the actual column if there are 6-in weirs.
6. What is the diameter of a packed column for this absorber with 1.5-in, ceramic Berl saddles at 75% of flooding?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





