Use the approach given in Example 20.11 to determine the length of tubes necessary to vaporize an
Question:
Use the approach given in Example 20.11 to determine the length of tubes necessary to vaporize an organic liquid (acetic acid at 1 bar) flowing inside a set of vertical ¾-in, BWG 16 tubes using condensing steam on the outside of the tubes to provide the energy for vaporization. The major resistance to heat transfer is expected to be on the inside of the tubes, and the wall temperature, as a first approximation, may be assumed to be at the temperature of the condensing steam, which for this case is 125°C. It may be assumed that the value of the vapor quality, x, varies from 0.05 to 0.95 in the tube. The physical parameters for acetic acid are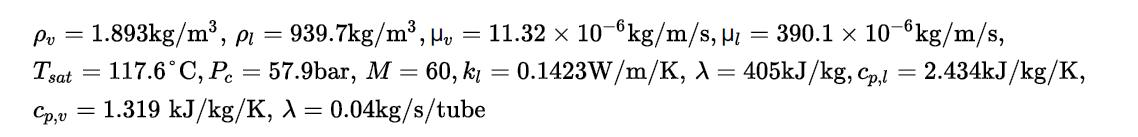
Example 20.11
Draw the T-Q diagram for the following cases:
Condensing (pure) vapor using cooling water
Distillation reboiler using condensing steam as the heating media
Process liquid stream cooled by a another process stream
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting




