Question: For the system shown in Fig. 11.30, compute the volume flow rate of ethyl alcohol at 77°F that would occur if the pressure in tank
For the system shown in Fig. 11.30, compute the volume flow rate of ethyl alcohol at 77°F that would occur if the pressure in tank A is 125 psig. The total length of pipe is 110 ft.
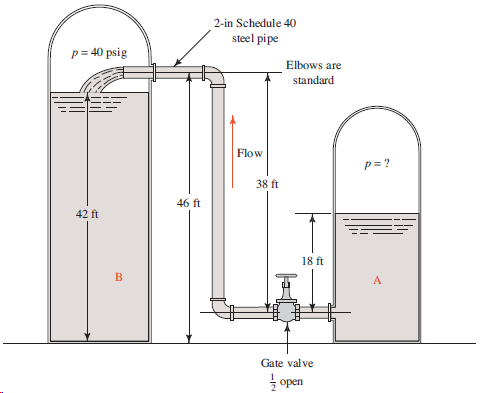
2-in Schedule 40 steel pipe p = 40 psig Elbows are standard Flow p= ? 38 ft 46 ft 42 ft 18 ft B Gate valve open
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
APPLIED FLUID MECHANICS Objective Volume flow rate Problem 1135 Figure 1130 System Data Pressure at ... View full answer

Get step-by-step solutions from verified subject matter experts


