Complete the table: Type of electrolyte (strong, weak or Substance Name nonelectrolyte) NaCl HNO3 Mg(NO3)2 HF NaF
Question:
Complete the table:
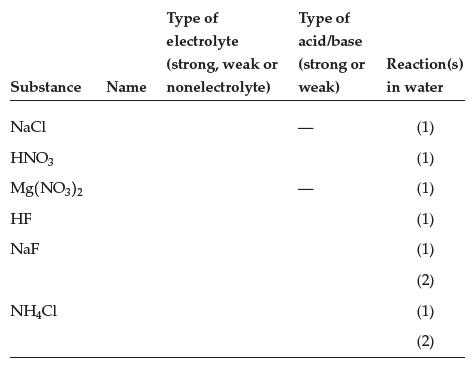
Transcribed Image Text:
Type of electrolyte (strong, weak or Substance Name nonelectrolyte) NaCl HNO3 Mg(NO3)2 HF NaF NH4Cl Type of acid/base (strong or weak) - Reaction (s) in water (1) ¤ e ¤ ¤ e d e ª
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Here is the completed table Substance Type of electrolyte Type of acidbase reac...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
For an ideal gas, the decrease in internal energy of 1.4 kgm is -343KJ when the volume increases from 0.043 cu. m. to 0.13 from 0.07 bar to 0.02 bar; c, = 0.72 cu. m. and the pressure decreases kgm-K...
-
Complete the table below for contribution margin per unit, total contribution margin , and contribution margin ratio: A Number of units 1,720 units 14,920 units 4,620 units Sales price per unit $...
-
Problem 13-1A Calculation and analysis of trend percents LO A1, P1 Selected comparative financial statements of Haroun Company follow. HAROUN COMPANY Comparative Income Statements For Years Ended...
-
Discuss types of media used in the design of a network given different situations. Namely: What would you do for a college campus with hundreds of staff users and students? How would they connect...
-
Witt Corporation received its charter during January 2011. The charter authorized the following capital stock: Preferred stock: 10 percent, par $10, authorized 21,000 shares Common stock : par $8,...
-
Complete the following feedback form on the outdoor treasure hunting experience to provide teachers with insight into how the experience went from an observer's perspective. ? Case Study 2b - Finding...
-
An increase in depreciation expense __________ earnings and __________ cash flow
-
A liquid storage system is shown below. The normal operating conditions are q 1 = 10 ft 3 /min, q 2 = 5 ft 3 /min, h = 4 ft. The tank is 6 ft in diameter, and the density of each stream is 60lb/ft....
-
Your grandmother left you an inheritance that pays $540 at the end of each year for a total of 16 consecutive years. However, because of your young age, the payments will not begin until the end of 3...
-
A 0.20 M aqueous solution of monoprotic acid HX has a pH of 2.14. (a) Is HX a strong acid or a weak acid? (b) Calculate K eq for the reaction of HX with water.
-
If 100 mL of an aqueous solution of nitric acid contains 0.030 mole of HNO 3 , what is the pH of the solution?
-
Goods with a list price of 37,500 were returned to the supplier. When purchased the supplier had allowed 5% trade discount and had offered 4% cash discount for settlement within a week. The amount to...
-
In addition to the strongest military in the world, the United States wields enormous soft power. Define soft power. What factors make the United States powerful when it comes to soft power?
-
Tampa by the Bay Cardiology practice is experiencing long wait times for new patient appointments. Next available appointment is 30 days. The administrator has asked the practice manager to construct...
-
In 2013, Idalia Hernndez Ramos, a middle school teacher in Mexico, was a victim of cyber harassment. After discovering that one of her students tweeted that the teacher was a "bitch" and a "whore,"...
-
Your life couldn't be any better. You just accepted a new role as a senior consultant for a project management services firm in San Francisco, and the move is finally happening. You've got a great...
-
What are the two "engines" that drive earth's processes, how do they work (basically) and what are their energy sources? How do the "engines" influence and interact with the Earth Systems? (provide a...
-
Kalyagin Investments acquired $220,000 of Jerris Corp., 7% bonds at their face amount on October 1, 2016. The bonds pay interest on October 1 and April 1. On April 1, 2017, Kalyagin sold $80,000 of...
-
5. How much would you need to deposit in an account now in order to have $5,000 in the account in 5 years? Assume the account earns 2% interest compounded monthly. 10. You deposit $300 each month...
-
Determine the equations of the elastic curve using the coordinates x 1 and x 2 , specify the slope and deflection at B. EI is constant. Thc A X2
-
Determine the deflection at B of the bar in Prob. 82. The bar is supported by a roller constraint at B, which allows vertical displacement but resists axial load and moment. If the bar is subjected...
-
The bar is supported by a roller constraint at B, which allows vertical displacement but resists axial load and moment. If the bar is subjected to the loading shown, determine the slope at A and the...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


