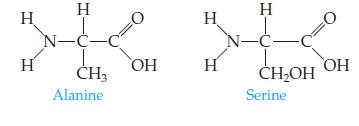
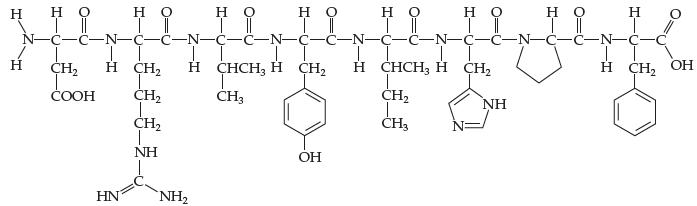
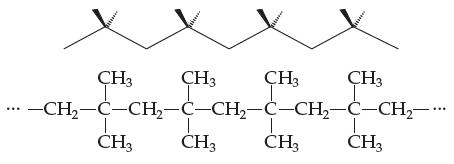
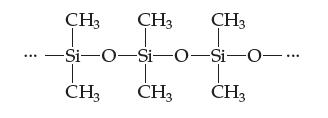
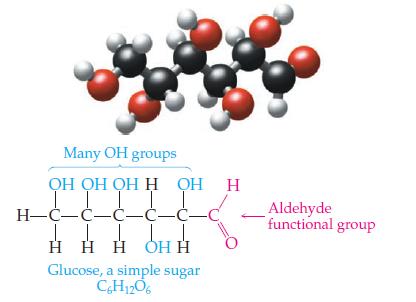
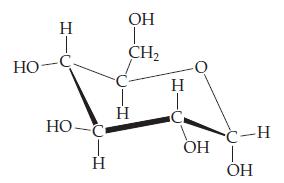
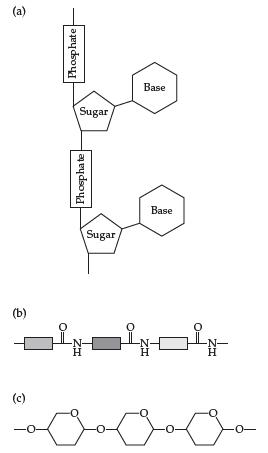
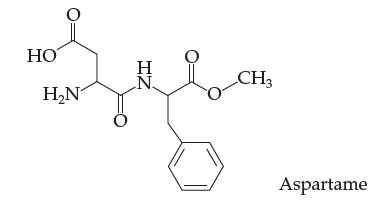
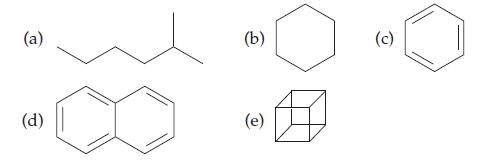
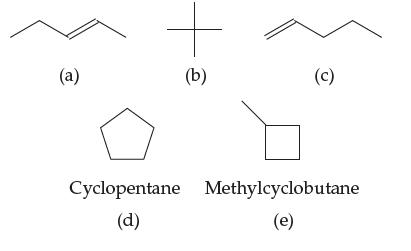
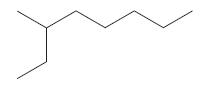
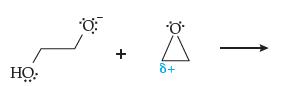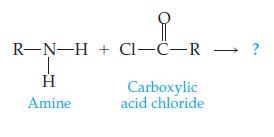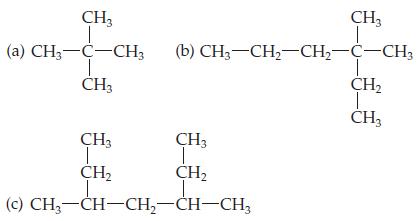Introductory Chemistry Atoms First 5th Edition Steve Russo And Michael Silver - Solutions
Unlock the comprehensive guide to mastering chemistry with our extensive collection of questions and answers from "Introductory Chemistry: Atoms First, 5th Edition" by Steve Russo and Michael Silver. Access the complete solution manual and answers key, providing step-by-step answers and solved problems to enhance your understanding. Explore chapter solutions and the instructor manual for detailed insights. Our online resource includes a test bank and solutions PDF for seamless learning. Enjoy the convenience of a free download of essential textbook solutions, making chemistry concepts clear and accessible for students and educators alike.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()