Question: For the tank shown in Fig. 3.21, determine the reading of the bottom pressure gage in psig if the top of the tank is vented
For the tank shown in Fig. 3.21, determine the reading of the bottom pressure gage in psig if the top of the tank is vented to the atmosphere and the depth of the oil h is 28.50 ft.
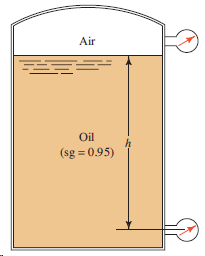
Air Oil (sg = 0.95)
Step by Step Solution
3.36 Rating (171 Votes )
There are 3 Steps involved in it
p h 095 624 ... View full answer

Get step-by-step solutions from verified subject matter experts


