Use Fig. 4.53. The surface is 1.50 m long. 2.80 m 1.20 m +: Water
Question:
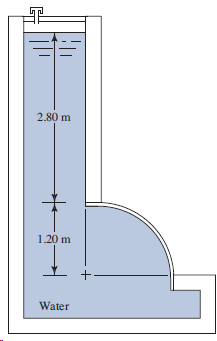
Transcribed Image Text:
2.80 m 1.20 m +: Water
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
F v Aw 981 3669 150 540 kN x 1 122 0...View the full answer

Answered By

Sandip Nandnawar
I am a B.E (Information technology) from GECA and also have an M.C.M from The University of RTMNU, MH.
I worked as a software developer (Programmer and TL). Also working as an expert for the last 6 years and deal with complex assessment and projects. I have a team and lead a team of experts and conducted primary and secondary research. I am a senior software engg and senior expert and deal with all types of CSE and IT and other IT-related assessments and projects and homework.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
As shown in Fig. P1.38, an underwater exploration vehicle submerges to a depth of 1000 ft. if the atmospheric pressure at the surface is 1 atm, the water density is 62.4 lb/ft3, and g = 32.2ft/s2,...
-
Use Fig. 4.52. The surface is 1.50 m long. Water 2,80 m 1.20-m radius
-
Use Fig. 4.50. The surface is 4.50 ft long. Oil sg = 0.85 9.50 ft -7.50 ft
-
Consider the following NLP problem: Maximize 2Xi + X2 - 2X3 + 3XiX2 + 1X subject to the constraints (a) Set up and solve the model by using Solver. Use a starting value of zero for each decision...
-
Punjab Company, Ltd. reported the following information for November and December 2017. ____________________________________November December Cost of goods...
-
Find the area under the normal curve that lies between the following pairs of z-values: a. z = -3.00 and z = 3.00 b. z(0.975) and z(0.025) c. z(o.io) and z(o.oi)
-
Glass as a waste encapsulant. Because glass is not subject to radiation damage, encapsulation of waste in glass is considered to be one of the most promising solutions to the problem of low-level...
-
Shown below is an excerpt from a citys subsidiary ledger for the rst two months of its scal year. Missing is the column that explains or references each of the entries. 1. Prepare the journal entries...
-
(After researching the different forms of business, Nat decides to operate Best Cookies Class as a proprietorship. Best Cookies Class offers services of conducting baking class of cakes and biscuits....
-
Bohrer, CPA (Chartered Professional Accountant), is considering the following factors in assessing audit risk at the financial statement level in planning the audit of Waste Remediation Services...
-
Use Fig. 4.51. The surface is 4.00 m long. Gasoline sg = 0.72 5.20 m 6,00 m -300
-
Use Fig. 4.54. The surface is 60 in long. 48 in 36 in Alcohol sg = 0.79
-
McGriff Dog Food Company normally takes 27 days to pay for average daily credit purchases of $9,530. Its average daily sales are $10,680, and it collects accounts in 32 days. a. What is its net...
-
Because her insurance agency is in a lakeside community, Adriana always asks her homeowners clients what boating activities they engage in, and she is sure to add the Watercraft endorsement...
-
1. Among all assumptions in CVP analysis, which one do you think is the most critical? Explain. 2. How will you change the cost-volume-profit analysis if the assumption (you identify in the previous...
-
Construct a confidence interval for p-P2 at the given level of confidence. x =26, n =229, x2 = 31, n = 302, 95% confidence The researchers are % confident the difference between the two population...
-
AP 9-2 (Moving Expenses) In May of the current year, following a dispute with her immediate superior, Ms. Elaine Fox resigned from her job in Halifax and began to look for other employment. She was...
-
Minimize the number of states in the following DFA: A b b a a a b b b E B a a
-
Describe elasticity of demand in your own words.
-
d. The characteristic equation of a control system is given by s+2s+8s+12s+20s+16+16=0. Determine the number of the roots of the equation which lie on the imaginary axis of s-plane
-
Draw a plausible mechanism for each of the following reactions: (a) (b) [H2SO4] Et,NH 'N' -H20 [H2SO4] Me-NH -H20
-
Predict the major product for each of the following reactions: (a) (b) [H*] N-H -H20 :? [] -H20
-
Predict the major product for each of the following intramolecular reactions: (a) (b) NH ? [H7 -H20
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...
-
Regarding Enron, this was a company that resulted in the creation of the Sarbanes-Oxley Act and many reforms to the accounting profession. Research the company and answer the following...

Study smarter with the SolutionInn App


