Add labels to the figure that follows, which illustrates the subatomic particles associated with a carbon atom.
Question:
Add labels to the figure that follows, which illustrates the subatomic particles associated with a carbon atom.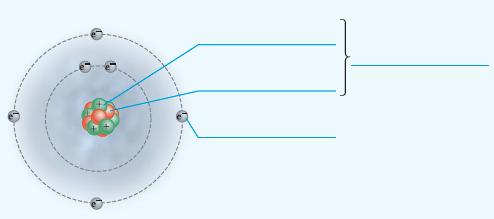
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Proton ...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

Biology Science For Life With Physiology
ISBN: 9780134555430
6th Edition
Authors: Colleen Belk, Virginia Maier
Question Posted:
Students also viewed these Sciences questions
-
Add labels to the figure that follows, which illustrates how Mycobacterium tuberculosis evolves when it is exposed to an antibiotic. Single drug therapy The initial M. tuberculosis population...
-
Add labels to the figure that follows, which illustrates a transfer RNA molecule. tRNA AAA UUU
-
Add labels to the figure that follows, which illustrates female internal reproductive organs.
-
In a test on 2000 electric bulbs, it was found that the life of a particular make was normally distributed with an average life of 2040 hos & S.D of bohos Estimate the ne of likely to burn for it...
-
Consider the fuel element of Example 5.9, which operates at a uniform volumetric generation rate of q) = 10 7 W/m3, until the generation rate suddenly changes to q2 = 2 x 107 W/m3. Use the...
-
Roulette is a game of chance that involves spinning a wheel that is divided into 38 equal segments. A metal ball is tossed into the wheel as it is spinning, and the ball eventually lands in one of...
-
What is the basic premise of ABC analysis? What are the three steps in making an ABC inventory analysis? LO.1
-
The Office Mart store in South Beach experienced the following events during the current year: 1. Incurred $400,000 in marketing costs. 2. Purchased $1,200,000 of merchandise. 3. Paid $40,000 for...
-
Stella's mortgage on her main home is $1 million and meets all qualifications for the mortgage interest deduction . She is a joint filer and paid $60000 in interest for 2023. Stella can claim the...
-
Water ___________. A. Is a good solute; B. Facilitates chemical reactions; C. Serves as an enzyme; D. Makes strong covalent bonds with other molecules; E. Consists of two oxygen and one hydrogen atoms
-
List the structural features in a prokaryotic cell.
-
You have just turned twenty-three years old, and are about to start working. Right now, you do not have any assets but have a student loan debt of $40,000. Your starting salary is w23 = $40,000 per...
-
At 3 1 st March, 2 0 2 3 , AB Ltd , had an Authorized Capital of K 3 5 , 0 0 0 divided into 1 0 , 0 0 0 7 . 5 % noncumulative per share being due on 3 0 th June, 1 9 6 4 . per share paid, the...
-
A Leadership and Workforce Development Perspective. The literature review should discuss the related literature, organized by topic or themes (not a list of sources). A literature review includes...
-
Critical Success Factors (CSF) are elements that are necessary for an organization or a project to attain its objectives. For example, Chief Executive support is a CSF for corporate sustainability...
-
Ultra Ceramic Products presented the following data for its operations for the month of October, 2020: Dept 1 Work in process, July t. 1(Conversion costs, 60%) 7,000 units Transferred to Dept 2 Work...
-
Choose a global organizational leader who demonstrated how a high level of ethical communication via social media technologies have worked best at building trust with virtual stakeholders. Identify a...
-
Virtual memory provides a mechanism for isolating one process from another. What memory management difficulties would be involved in allowing two operating systems to run concurrently? How might...
-
1. Below is depicted a graph G constructed by joining two opposite vertices of C12. Some authors call this a "theta graph" because it resembles the Greek letter 0. a. What is the total degree of this...
-
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
-
Liquid N 2 has a density of 875.4 kg m 3 at its normal boiling point. What volume does a balloon occupy at 298 K and a pressure of 1.00 atm if 3.10 10 3 L of liquid N 2 is injected into it? Assume...
-
Calculate the volume of all gases evolved by the complete oxidation of 0.375 g of the amino acid alanine NH 2 CHCH 3 COOH if the products are liquid water, nitrogen gas, and carbon dioxide gas, the...
-
Mediocre Company has sales of $120,000, fixed expenses of $24,000, and a net income of $12,000. If sales rose 10%, the new net income would be: Question 18 options: $16,800 $36,000 $13,200 $15,600
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...

Study smarter with the SolutionInn App


